Molarity
advertisement

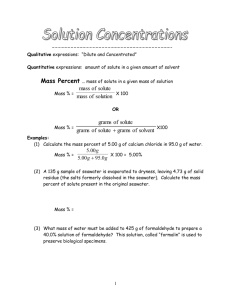
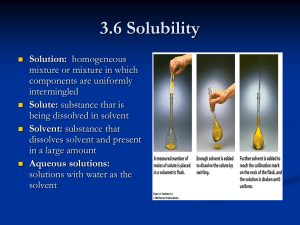
Sec 5.6 - Molarity A solution is formed when 1 “thing”, a solute, is dissolved in another “thing”, a solvent. Water is called the universal solvent because it is capable of dissolving many things. A solution can be a liquid in a liquid, solid in a liquid, (iced tea), gas in gas or solid in solid. The strength of a solution needs to be measured. It is not good enough to say that the iced tea is strong, we need to say how strong. There are several ways to describe the concentration of a solution: Percent by volume, ex; 40% alcohol Grams per liter, ex; 20 g of salt in 1 liter of water The best way is molarity! The molarity of a solution is the number of moles of the solute divided by the volume of the solvent, in liters. To figure out the molarity of a solution, use the molecular weight and simply work out the number of moles of the solute. Then divide by the volume of the solvent. molarity (M) of the solution = moles solute liters of solvent Example 1 If you have 10.0 grams of NaCl, table salt, and dissolve it in 500.0 ml of water, what is the molarity of the solution? Example 2 Suppose you had 58.44 grams of Ba(NO3)2 and you dissolved it in exactly 2.00 L of solution. What would be the molarity of the solution? Preparing Molar Solutions In order to prepare a molar solution with an exact molarity, you will need to use a beaker, wash bottle, electronic balance and volumetric flask. Using Molarity to calculate the amount of grams needed to make the solution 1. Calculate the grams of KBr required to make 500.0 mL of a 0.400M solution. 2. Outline the procedure to Prepare a 0.050M solution of CuCl2 in a 250.0mL volumetric flask. Steps 1. 2. 3. 4. weigh out ________ of the solid on a electronic balance dissolve the solid in the beaker transfer it to a volumetric flask add distilled water up to the mark HW pg 98 # 59, 60c, g, 61-64 PRACTICE: How many grams of CaCO3 are required to make 250.0mL of 0.150M solution? Sec 5.6 Dilutions Calculations M1V1 = M2V2 M1 – the original molarity V1 – original volume M2 – New molarity after mixing V2 – new volume after mixing Example If 300.0 mL of 0.15M CaCl2 is added to 100.0mL of H2O, what is the new [CaCl2]? How many litres of a 15.4M HNO3 solution is required to make 2.50L of a 0.375M solution? Mixing 2 solutions with common ions If 300.0 mL of 0.250M NaCl is added to 500.0mL of 0.100M NaCl, what is the new [NaCl]? HW pg 102 # 78-82, 84, 87, 88, 90, 91 PRACTICE 450.0mL of 0.125M CaCl2 is mixed with 175.0mL of 0.761M CaCl2. What is the new concentration of CaCl2