Triazine Herbicides
advertisement

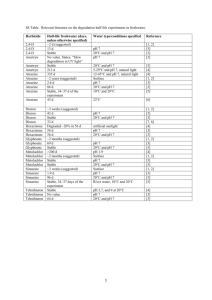
Triazines and Triazoles The triazines are among the largest groups of herbicides, second only to the acetamides in the number of commercial compounds. The closely related triazoles also include compounds used as agricultural and pharmaceutical fungicides, and even as insecticides. Finally, at least one triazone compound is used pharmaceutically, in anticancer chemotherapy. It functions as an alkylating agent. Triazines can be synthesized by reacting cyanuric chloride with the appropriate alkylamine(s) in the presence of acid acceptors. The triazine ring show remarkable stability to natural degradative processes: neither biological degradation nor photolysis lead to significant decomposition of the ring structure at ambient temperatures. Herbicidal activity allows relatively few substituents at the 2-carbon position: Cl, OCH3 or SCH3 are the most common. Of these, Cl results in lower water solubility than either OCH3 or SCH3. Substitution at C-4 and C-6 can be more varied without destroying herbicidal activity, but N-alkyl substituents are most common. Thus atrazine, for example, has Cl at the C-2 position, with N-ethyl and N-isopropyl substituents at C-4 and C-6. All of the triazines share a single mechanism for their herbicidal activity: inhibition of the Hill reaction of photosynthesis. Nonetheless, different species of plants differ in their sensitivity to individual triazines, and the individual triazines differ in their phytotoxicity. Thus atrazine is most commonly used as a selective herbicide, whereas simazine is frequently used as a soil sterilant, although it is also used on crops. Amitrole (Figure 1) is not actually a triazine; rather, it is a triazole, with a 5-atom heterocyclic ring. It shares many of the properties of the triazines, however: including not only the mechanism of herbicidal action, but also the penchant for controversy that has marked triazines ever since they came on the market. Figure 1: Amitrole Amitrole was introduced in the late 1950s for post-herbicide use on cranberries. This is an unusual crop because it has essentially only one market period: Thanksgiving. Thirteen days before Thanksgiving of 1959, the Food and Drug Administration (FDA) announced that it had seized a large part of that year’s cranberry crop because it contained residues of amitrole, which had been shown to cause cancer in animals. The Delaney Clause, part of the 1958 amendment of the Food and Drug Act, states that no chemical that has been shown to cause cancer in humans or animals may be added to food. Pesticide application is considered as ‘adding to’, so FDA had no choice but to take the contaminated cranberries off the market. It was a sufficiently large part of the crop that the initial concern was whether there would be enough cranberries for the Thanksgiving market. Unfortunately FDA’s action spawned a panic among consumers, leading to a drastic decrease in the sale of cranberries of any kind that year. As a result, even though a large part of the harvest was later released as uncontaminated, the incident remains a by-word among both pesticide toxicologists and farm groups. Unlike most pesticides (including the triazines), amitrole is a highly water-soluble compound (280,000 ppm) and essentially insoluble in non-polar solvents. It is nonselective in fallow soils – meaning it will kill all growth. Its herbicidal action is to interfere with photosynthesis by inhibiting the Hill reaction. Amitrole is not acutely very toxic to mammals: its median lethal dose is well over 1 gm/kg in all species. In repeated exposures it is more active: 1,000 ppm in the diet1 will cause enlarged thyroids in rats within 3 days, and chronic dosing at 60-120 ppm in the diet will eventually lead to enlarged thyroids. It does not appear to be mutagenic, but increases the incidence of thyroid adenomas2, adenocarcinomas and hepatomas in both rats and mice, and the incidence of pituitary tumors in rats. It also inhibits cytochromes P450. I. Cyanazine (Figure 2) is a commonly used triazine with a water solubility more typical of the class (although at the high end): 171 ppm. It is stable to UV light, but hydrolyzed by pH less than 5 or greater than 9. It has a relatively low LD50 of 334 mg/kg, if given po to rats. The dermal LD50 in rabbits is 2,000 mg/kg which, although high enough not to be a major concern, is low for this class. High doses produce lethargy, inactivity, and depression in animals. There have been almost no reports of human toxicity due to cyanazine exposure. And, in contrast to many other triazines, cyanazine is negative in the whole battery of in vitro (cell-based) mutagenesis assays, as well as in the in vivo carcinogenesis assay run in mice. It is, however, a developmental toxicant, causing birth defects in rats at doses as low as 25 mg/kg/day, with a calculated NOAEL of 1 mg/kg. Fetal death occurs in rabbits at doses of 2 mg/kg/day. This developmental toxicity has led to restrictions on its use. Figure 2: Cyanazine Simazine (Figure 3) is the most persistent – and least water-soluble – of the commonly used triazines. It is soluble in water to 5 ppm, and has a 2-8 month persistence in Illinois soils. Like atrazine, it is more persistent in dry soils, but its persistence even under ordinary conditions of soil moisture is great enough that it is not used on corn because of the high probability that it would carry over into the next season, and damage the next season’s soybeans( which are typically planted after corn). It is most often used as a soil sterilant, but also has crop uses. Until 1992 it was also used to control submerged aquatic weeds. 1 In rodents, 10 ppm in the diet corresponds roughly to 1 mg/kg given po. Hepatomas and adenomas are benign tumors; adenocarcinomas are malignant. With the exception of a few benign tumor types that never become malignant, it is prudent to assume that benign tumors are early stages in the development of cancer. 2 Figure 3: Simazine Simazine shares the common herbicidal activity of the class, interfering with the Hill reaction of photosynthesis. Its oral LD50 in rats is > 5,000 mg/kg; its dermal LD50 in rabbits is > 10,000 mg/kg. Sheep are somewhat more susceptible: the oral LD50 is approximately 500 mg/kg, and doses can be cumulative: e.g., a total dose of 1,400 mg/kg can be lethal. Symptoms of nonfatal simazine poisoning in mammals include tremors, convulsions, hindlimb weakness, decreased food intake and increased water intake. In chronic dosing, simazine increased the incidence of dominant lethals in Drosophila melanogaster, but was negative in all other mutagenesis assays. Similarly, it increased the incidence of mammary tumors in female rats, but was negative in the mouse assay. Therefore its mutagenicity and carcinogenicity are disputed. There is one study that suggests an increased prostate cancer risk among farmers exposed to simazine. Atrazine (figure 4) is a pre- and post-emergent herbicide, introduced in 1957 by Geigy Chemical Company. It is a white crystalline solid with a melting point of 173-175oC and a water solubility of 33 ppm at 27oC. It is soluble in methanol at 18,000 ppm, and in chloroform at 52,000 ppm. Atrazine is hydrolyzed by alkali or mineral acids at higher temperatures, but is stable to mild acid or base. Atrazine is used for general weed control and (at somewhat lower levels) for selective weed control in sorghum, sugar cane, pineapple and corn. Its use in midwestern corn fields makes it the second most heavily used herbicide in the United States, with annual use of almost 80 million pounds by the mid-1980s. Figure 4: Atrazine The major degradative process undergone by atrazine in soil is its conversion to hydroxyatrazine by dechlorination. Much of this results from attack by microorganisms, since atrazine degrades significantly more slowly in sterile soil, and more rapidly in soil to which it has been repeatedly applied. Hydroxyatrazine is also the major degradative product of photolytic decomposition of atrazine in water. Both of the N-alkyl chains are also subject to microbial attack. There is, however, no evidence that microorganisms can break down the triazine ring, and several studies suggest that even side chain breakdown is not readily achieved when other sources of carbon are available to the microorganisms. Similarly, very few metabolites of atrazine, and no ring cleavage, has been identified in animals. Atrazine does not, however, accumulate in animal tissues, nor is it excreted in milk (a serious consideration because of its presence in much cattle feed). As suggested by the minimal evidence for degradation, atrazine is relatively persistent for a modern pesticide. Many studies of atrazine persistence deal with its herbicidal activity rather than with persistence of chemical moieties; even those that deal with chemical persistence assume that the herbicide will not only be at agriculturally relevant levels, but that it will be present in the plow and root layers of the soil, accessible to microbial action. It has been estimated that, in sterile soil at 20oC, atrazine would have a half life of 9 to 116 years: a range that clearly points up the uncertainties in the estimate. Certainly atrazine persistence is inversely correlated with the amount of rainfall and the temperature, but also depends on soil type. It disappears more rapidly from sandy soils than from clays due to leaching, but persists longer in coarse than in fine soils. Transport through soil is inversely correlated with its adsorption to soil, and especially to the organic fraction of soil. Atrazine is found in much of the surface water of corn-growing areas, at least in the spring, when it runs off fields to which it has been applied as treatment against corn rootworms (Diabrotica species). Allowable levels of atrazine in groundwater are 3 ppb, but higher levels are found so frequently that the manufacturer is seeking to have these levels raised. At present (1999), levels of 30 ppb are common in Illinois streams in late spring, with occasional peaks running even higher. Because so much of the Illinois population obtains its drinking water from surface reservoirs or shallow aquifers, a majority of drinking water systems tested (116 in 54 counties, serving 1,400,000 people) contain herbicides; atrazine is the most common.3 Over 70% of all Illinois drinking water samples tested positive for atrazine from 1990 to 1992. There were 48 systems, serving 524,000 people, for which at least one test showed herbicide levels above legal limits.4 It has been known since the late 1960s that atrazine will reach groundwater. In 1974 both atrazine and its metabolites were identified in the drinking water of New Orleans5. The larger fraction of the atrazine that reaches water is soil-associated. Thus any tillage practice that reduces soil erosion will also decrease atrazine contamination of water, and especially of surface water. It must be emphasized, however, that atrazine is somewhat soluble in water, and does reach groundwater. Moreover, because of its resistance to nonbiological degradation, it is very persistent in groundwater once it is present at all. And, as detection methods have improved, more and more groundwater in the Midwest has been shown to contain atrazine residues. This has fueled a major controversy over the significance of such contamination. The major question is whether or not atrazine has toxic properties that do not allow setting of thresholds for toxicity: in practice (since atrazine is not very toxic to mammals in terms of acute toxicity, below), this means that the controversy hinges on atrazine's genotoxicity. 3 Chicago obtains its water from Lake Michigan, and atrazine is fond in all 5 Great Lakes, so there may well be traces of atrazine in Chicago's drinking water. In contrast, the Champaign-Urbana water supply, which originates in the very deep Teays aquifer, is uncontaminated by herbicides. 4 Note that this means that the majority of systems did not exceed legal limits. However, 12 systems had average annual levels of at least one herbicide exceeding legal limits. The largest of these systems was that of Springfield. 5 Anonymous, 1974. Draft Analytical Report. New Orleans Area Water Supply. U.S. Environmental Protection Agency Region VI Dallas TX. 28 pp. Atrazine is taken up by plants of all species. In sensitive species, it inhibits the Hill reaction of photosynthesis, leading to death of the plant. Its selectivity depends on the resistance of some plant species (notably monocots) to its action, rather than to differences in uptake. The acute toxicity of atrazine to mammals is low. Its oral LD50 to rats is between 2,000 and 3,000 mg/kg, and there is little cumulative toxicity to repeated dosing. Dietary levels of 100 or 500 mg/kg/day caused growth retardation in rats, reportedly because the rats ate less. Symptoms after a single nearlethal dose include respiratory excitation followed, by depression; hypothermia, and motor incoordination. Following a single lethal oral dose, death occurs within 24 hours. Pathologically, lung edema and hemorrhages of numerous organs are seen. The Ld50 following dermal dosing in rabbits is greater than 3,000 mg/kg. Atrazine does not appear to cause malformations in rodents, but does cause fetal death at maternally lethal doses in sheep. Fetal deaths have not been reported in any species at doses that were not maternally toxic. These data argue for a negligible level of developmental toxicity. The genotoxicity of atrazine is well established, as long as one includes the activity of its metabolites. In some in vitro systems, notably those that do not include eukaryotic metabolic capacities, atrazine does not cause DNA damage. In all eukaryotic systems that allow metabolism, DNA damage is seen. Thus, corn plants (which are resistant to atrazine) metabolize atrazine to a DNA-damaging metabolite. Atrazine is actually carcinogenic in rodents, with increases in tumors seen in two strains of rats6 as well as in mice. To interpret these data, one must consider the meaning of a positive animal bioassay. Animal tests for carcinogenesis are typically carried out in 2 species, and in both sexes of each species. For each dose, 20 animals of each sex within each species are treated for most of their lives (beginning soon after weaning). Typically, 3 levels of treatment as well as vehicle treated controls are run. These animals serve to model the risk posed by low levels of environmental contaminants - say, atrazine at 3 ppb in drinking water. The upper bound of acceptable risk (that is, the level of risk that triggers regulatory action) is currently at 1 cancer death per million people. Thus dosing of 20 animals per sex per species must determine whether a chemical will increase cancer deaths by 1 in a million. Clearly this cannot be achieved by using realistic levels of exposure. Therefore the animals are exposed to the maximum tolerated dose: the highest dose that does not cause adverse noncancer effects over the period of dosing. The incidence of tumors is determined separately for every organ, and the risk extrapolated downward to relevant exposures. Safety factors are then worked in to assure that differences in species sensitivities do not put humans at greater than tolerable risk. One of the problems with animal assays is that the method of extrapolation used to estimate the risk from environmental exposure makes a huge difference. Consider animals dosed at the maximum tolerated dose for a carcinogenesis study, Figure 5a. Levels of exposure are very low relative to the administered doses (which are used because we do not have resources to run ‘megamouse’ studies at realistic doses.) The usual extrapolation is straight line (Figure 5b), either to the origin, or to the level of tumors in control animals (it is assumed that control animals adequately model the background incidence of tumors). This method gives a somewhat higher risk at low levels of exposure than does a 6 These data, like those of many cancer studies, are not completely unequivocal. The results in Fischer rats have been interpreted as positive (by the original authors) and as negative (in a reanalysis by scientists working for Ciba-Geigy, which manufactures atrazine). curvilinear extrapolation (Figure 5c). For theoretical reasons7 it is assumed that there is no threshold, so the models shown in Figure 5d and 5e are not used % R e s p o n s e x x x Log Dose Figure 5a % R e s p o n s e Linear response; no threshold x x x Log Dose Figure 5b 7 Given what we know about mechanisms of carcinogenesis, we assume that a single molecule of a mutagen could initiate the process. This is in direct contradiction to the underlying axiom of classical toxicology, which is that, for each chemical, there is a threshold below which there will be no toxic effects. Nonlinear response, no threshold % R e s p o n s e x x x Log Dose Figure 5c Linear response with threshold % R e s p o n s e x x x Log Dose Figure 5d Nonlinear response with threshold % R e s p o n s e x x Log Dose Figure 5e x In contrast to acute toxicity, the delayed toxicities of a chemical are often hard to determine. The longer the delay between exposure and appearance of symptoms, the greater the difficulty. As time passes, people forget what they did, what they worked with, what they were exposed to. Moreover, the less common a consequence, the harder it is to connect it to its cause. Even powerful carcinogens cause cancer in only a small fraction of exposed people. Cigarette smoking is the leading environmental cause of cancer, yet it causes lung cancer in only 10% of ‘heavy smokers’ (defined as those who smoke a pack a day for 20 years). Many carcinogens, at realistic environmental levels, will cause 2-3 cases per million exposed persons.