SCI 光盘版检索证明操作指南
注:SCI 光盘版检索证明模板在本文档最后一页
关于 SCI 光盘版论文检索收录证明
我建议要开具 SCI 光盘版论文检索收录检索证明的同学都先在 SCI 网络版数
据库上面搜索出自己的要出具证明的论文,确定好对应的收录年,并且留意 SCI
网络版中作者的表示方式,等下在进行检索时可能会用到的。
# SCI 光盘版链接:http://202.38.232.84/eresources/edetail.php?id=163
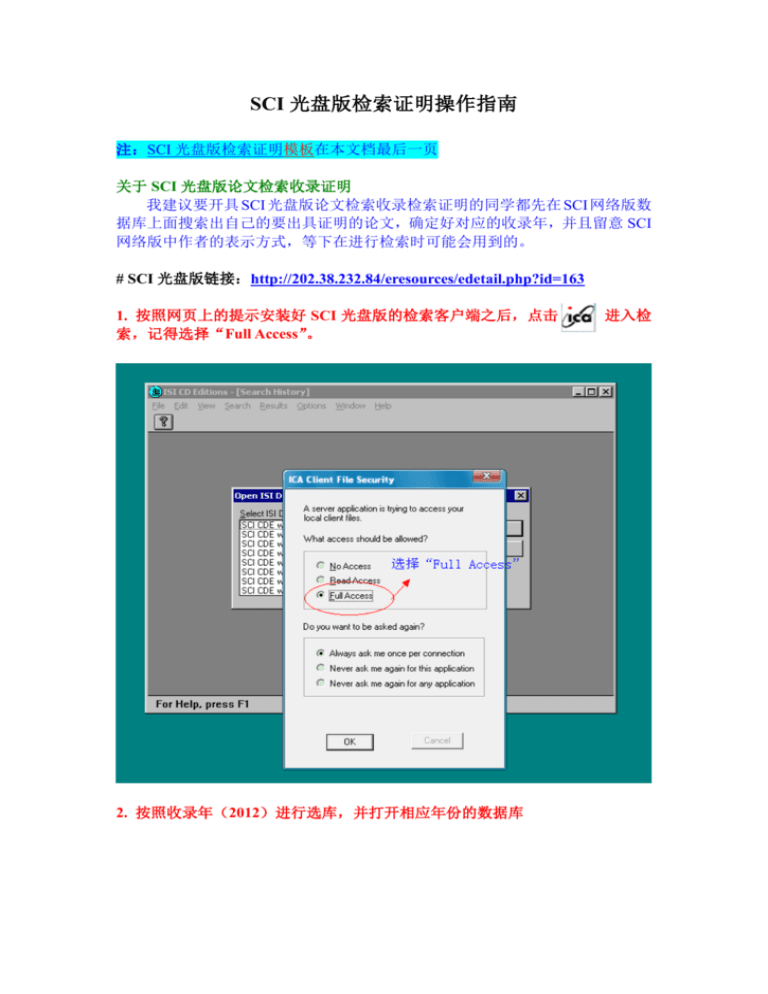
1. 按照网页上的提示安装好 SCI 光盘版的检索客户端之后,点击
索,记得选择“Full Access”。
2. 按照收录年(2012)进行选库,并打开相应年份的数据库
进入检
3. 确定检索的字段,进行检索
在 SCI 光盘版检索系统中不支持复制粘贴,检索词的输入只能依靠键盘一
个一个敲进去,不怕麻烦和打字速度快的同学可以选择题目字段(“Title Word”)
进行检索,想偷懒的同学可以选择作者姓名(“Author name)字段进行检索,要
求跟 SCI 网络版的要求一样(姓为全称,名取拼音首字母),为了提高检索效率,
最好使用 2-3 个作者的名称来检索,如果进行过网络版检索的同学就可以根据网
络版中的作者表示方法进行查找。
选择检索字段
输入检索词进行检索(最好用 2-3 个作者名称来查,记得作者字段检索词要求
是:姓为全称,名取拼音首字母)
打开检索结果
查找出自己需要的论文
4. 保存检索结果
保存检索结果
保存检索结果,需要注意的是保存的位置不能为 c 盘
5. 根据检索证明模板的要求,完成检索证明证明页的编辑
打开保存好的 txt 文件,将论文的有关信息按照 SCI 光盘版收录检索证明的
模板格式复制填入。
txt 文件中的论文详细信息
将论文的有关信息按照 SCI 光盘版收录检索证明的模板格式复制填入:
提示:SCI 光盘版检索证明分为:证明页和附件两个部分
SCI 光盘版检索证明证明样例
填入论文信息到模板中
SCI 光盘版收录证明的附件
完成信息的录入和格式编辑的编辑后,那就可以把文件打印出来到我们图书
馆情报咨询部(图书馆三楼 310 室)进行盖章,一份有效力的检索证明就完成了。
后附:SCI 光盘版检索证明模板
检索证明
根据委托方提供的论文目录(2012年)
,经美国SCI光盘数据库检
索,华南理工大学轻工与食品学院刘博(Liu-B)发表的论文被美国《科
学引文索引》SCI光盘版(2012年)收录了2篇(第一作者2篇;详情
见附件)。题录如下:
1. Authors: Liu-B Wang-XY Li-XY Zeng-XJ Sun-RC Kennedy-JF
Title:
RAPID
EXFOLIATION
OF
RECTORITE
IN
QUATERNIZED
CARBOXYMETHYL CHITOSAN
Full source: CARBOHYDRATE POLYMERS 2012, Vol 90, Iss 4, pp 1826-1830
Language: English
Document type: Article
Addresses:
S-CHINA-UNIV-TECHNOL, SCH LIGHT IND & FOOD, STATE KEY LAB PULP &
PAPER ENGN, GUANGZHOU 510640, GUANGDONG, PEOPLES-R-CHINA
S-CHINA-UNIV-TECHNOL, SCH MAT SCI & ENGN, GUANGZHOU 510640,
GUANGDONG, PEOPLES-R-CHINA
BEIJING-FORESTRY-UNIV, INST BIOMASS CHEM & TECHNOL, BEIJING 100083,
PEOPLES-R-CHINA
UNIV-BIRMINGHAM, SCH CHEM, BIRMINGHAM CARBOHYDRATE & PROT
TECHNOL GRP, BIRMINGHAM B15-2TT, W-MIDLANDS, ENGLAND
2. Authors: Liu-B Wang-XY Yang-B Sun-RC
Title: MICROWAVE-ASSISTED SYNTHESIS OF QUATERNIZED CARBOXYMETHYL
CHITOSAN IN AQUEOUS-SOLUTION AND ITS THERMAL-BEHAVIOR
Full source: JOURNAL OF MACROMOLECULAR SCIENCE PART A-PURE AND APPLIED
CHEMISTRY 2012, Vol 49, Iss 3, pp 227-234
Language: English
Document type: Article
Addresses:
S-CHINA-UNIV-TECHNOL, STATE KEY LAB PULP & PAPER ENGN, GUANGZHOU
510640, GUANGDONG, PEOPLES-R-CHINA
CHINESE-ACAD-SCI, GUANGZHOU INST GEOCHEM, STATE KEY LAB ORGAN
GEOCHEM, GUANGZHOU, GUANGDONG, PEOPLES-R-CHINA
BEIJING-FORESTRY-UNIV, INST BIOMASS CHEM & TECHNOL, BEIJING 100083,
PEOPLES-R-CHINA
特此证明
华南理工大学图书馆
信息咨询部
2013 年 4 月 23 日
附件:
华南理工大学轻工与食品学院刘博(Liu-B)发表的论文被美国
《科学引文索引》SCI 光盘版(2012 年)收录情况:
SCI CDE with Abstracts (Jan 12 - Dec 12)
(D4.0)
Record 1 of 2.
Authors: Liu-B Wang-XY Li-XY Zeng-XJ Sun-RC Kennedy-JF
Title: RAPID EXFOLIATION OF RECTORITE IN QUATERNIZED CARBOXYMETHYL
CHITOSAN
Full source: CARBOHYDRATE POLYMERS 2012, Vol 90, Iss 4, pp 1826-1830
Language: English
Document type: Article
IDS/Book No.: 013UI
No. Related Records: 20
No. cited references: 20
Addresses: S-CHINA-UNIV-TECHNOL, SCH LIGHT IND & FOOD, STATE KEY LAB PULP
& PAPER ENGN, GUANGZHOU 510640, GUANGDONG, PEOPLES-R-CHINA
S-CHINA-UNIV-TECHNOL, SCH MAT SCI & ENGN, GUANGZHOU 510640,
GUANGDONG, PEOPLES-R-CHINA
BEIJING-FORESTRY-UNIV, INST BIOMASS CHEM & TECHNOL, BEIJING
100083, PEOPLES-R-CHINA
UNIV-BIRMINGHAM, SCH CHEM, BIRMINGHAM CARBOHYDRATE &
PROT TECHNOL GRP, BIRMINGHAM B15-2TT, W-MIDLANDS, ENGLAND
Author
keywords:
EXFOLIATION;
NANOCOMPOSITE;
QUATERNIZED
CARBOXYMETHYL CHITOSAN; RECTORITE
KeyWords Plus: NANOCOMPOSITE; BEHAVIOR; DERIVATIVES; MICROWAVE
Abstract:
Exfoliated quaternized carboxymethyl chitosan/rectorite (QCMC/REC)
nanocomposite was prepared via microwave irradiation method for 70 min, which was performed
in only water without any additional plasticizer. XRD, TEM, AFM, SEM and FTIR results
revealed that when the mass ratio of QCMC to REC was no less than 4:1, the silicate layers of
REC were completely exfoliated in QCMC matrix and were homogenous with QCMC, the surface
of QCMC/REC nanobiocomposite was very smooth: two types of interactions of hydrogen bond
and electrostatic attraction existed in the QCMC/REC nanobiocomposite. Thermal analysis
indicated that QCMC/REC nanobiocomposite had higher thermal stability than only QCMC.
Therefore, the microwave irradiation method appears to be a promising tool for preparing
exfoliated biopolymer/layered silicate nanocomposites at a mild condition. (C) 2012 Elsevier Ltd.
All rights reserved.
Cited references:
BITINIS-N-2011-ADV-MATER-V23-P5229
CHIVRAC-F-2008-BIOMACROMOLECULES-V9-P896
CHIVRAC-F-2010-CARBOHYD-POLYM-V79-P941
GUO-ZY-2008-CARBOHYD-POLYM-V73-P173
HAN-YS-2010-J-PHYS-CHEM-SOLIDS-V71-P464
KABIRI-K-2010-J-APPL-POLYM-SCI-V116-P2548
LIU-B-2012-J-MACROMOL-SCI-A-V49-P227
MENG-XY-2009-J-APPL-POLYM-SCI-V113-P678
MUZZARELLI-RAA-1984-CARBOHYD-RES-V126-P225
MUZZARELLI-RAA-1987-CARBOHYD-POLYM-V7-P87
RATANAKAMNUAN-U-2012-CARBOHYD-POLYM-V87-P84
RAY-SS-2003-PROG-POLYM-SCI-V28-P1539
RUIZHITZKY-E-2011-CHEM-SOC-REV-V40-P801
SONG-YB-2012-J-MATER-CHEM-V22-P2512
WANG-X-2008-CHINESE-CHEM-LETT-V19-P37
WANG-XY-2007-CARBOHYD-POLYM-V69-P41
WANG-XY-2010-COMPOS-SCI-TECHNOL-V70-P1161
WANG-ZB-2008-APPL-CLAY-SCI-V42-P146
XU-T-2010-CARBOHYD-POLYM-V81-P931
ZHANG-XR-2007-MATER-LETT-V61-P1478
Record 2 of 2.
Authors: Liu-B Wang-XY Yang-B Sun-RC
Title: MICROWAVE-ASSISTED SYNTHESIS OF QUATERNIZED CARBOXYMETHYL
CHITOSAN IN AQUEOUS-SOLUTION AND ITS THERMAL-BEHAVIOR
Full source: JOURNAL OF MACROMOLECULAR SCIENCE PART A-PURE AND APPLIED
CHEMISTRY 2012, Vol 49, Iss 3, pp 227-234
Language: English
Document type: Article
IDS/Book No.: 916CX
No. Related Records: 20
No. cited references: 32
Addresses: S-CHINA-UNIV-TECHNOL, STATE KEY LAB PULP & PAPER ENGN,
GUANGZHOU 510640, GUANGDONG, PEOPLES-R-CHINA
CHINESE-ACAD-SCI, GUANGZHOU INST GEOCHEM, STATE KEY LAB
ORGAN GEOCHEM, GUANGZHOU, GUANGDONG, PEOPLES-R-CHINA
BEIJING-FORESTRY-UNIV, INST BIOMASS CHEM & TECHNOL, BEIJING
100083, PEOPLES-R-CHINA
Author keywords: AQUEOUS SOLUTION; MICROWAVE IRRADIATION; QUATERNIZED
CARBOXYMETHYL CHITOSAN; THERMAL STABILITY
KeyWords Plus: ANTIBACTERIAL ACTIVITY; ANTIMICROBIAL ACTIVITY;
MOLECULAR-WEIGHT; IRRADIATION; WATER; PROPERTY
Abstract:
A novel promising polyampholyte, being water-soluble quaternized carboxymethyl
chitosan (QCM-chitosan), can be prepared by grafting carboxymethyl groups and quaternary
ammonium groups on chitosan. Traditionally, QCM-chitosan was obtained by a conventional
heating method in organic solvent for a lengthy time. The present study was to prepare
QCM-chitosan rapidly under microwave irradiation for 70 min; the whole preparation proceeded
in water without any organic solvent. Firstly, chitosan was carboxymethylated, and sequentially
carboxymethyl chitosan (CM-chitosan) was quaternized to obtain QCM-chitosan. QCM-chitosan's
structure was characterized. GPC-LLS were used to analyze its weight - average molecular weight
(M-w), and the degree of substitution (DS) was determined by titration. The thermal stability of
QCM-chitosan was measured by TGA. The results proved that QCM-chitosan was synthesized.
The maximum degree of substitution (DS) of carboxymethyl groups and quaternary ammonium
groups under the optimum reaction condition was 0.82 and 0.48, respectively. Compared to
chitosan, the thermal stability of QCM-chitosan decreased because of disintegration of
intermolecular hydrogen bonding and partial breaking of the molecular structure, moreover, as DS
of quaternary ammonium and carboxymethyl groups increased, QCM-chitosan showed lower
thermal stability. In summary, the microwave irradiation method is a rapid and environmentally
friendly process to synthesize soluble chitosan derivatives.
Cited references:
CAI-ZS-2007-POLYM-BULL-V59-P655
CAI-ZS-2009-POLYM-BULL-V62-P445
CAI-ZS-2010-J-APPL-POLYM-SCI-V118-P299
CAO-ZB-2008-J-BIOMED-MATER-RES-A-V85-P99
CHOUDHARI-SK-2009-J-COLLOID-INTERF-SCI-V338-P111
DEVLIEGHERE-F-2004-FOOD-MICROBIOL-V21-P703
FENG-T-2008-CARBOHYD-POLYM-V73-P126
GASPAR-VM-2011-NANOTECHNOLOGY-V22-P1510
GE-HC-2005-CARBOHYD-RES-V340-P1351
GE-HC-2006-CARBOHYD-POLYM-V66-P372
GUO-ZY-2008-CARBOHYD-POLYM-V73-P173
KRISHNAPRIYA-KR-2009-CARBOHYD-RES-V344-P1632
KUMAR-S-2009-J-MACROMOL-SCI-A-V46-P1095
LEWANDOWSKA-K-2009-THERMOCHIM-ACTA-V493-P42
LI-HB-2004-COLLOID-SURFACE-A-V242-P1
LI-J-2006-J-APPL-POLYM-SCI-V102-P1098
LIANG-XF-2008-ACTA-CHIM-SINICA-V66-P2178
LIANG-XF-2008-LANGMUIR-V24-P7147
LIANG-XF-2010-J-NANOPART-RES-V12-P1723
LIDSTROM-P-2001-TETRAHEDRON-V57-P9225
LIU-L-2004-CARBOHYD-POLYM-V57-P97
LUO-JW-2010-J-MACROMOL-SCI-A-V47-P952
MUZZARELLI-RAA-1984-CARBOHYD-RES-V126-P225
MUZZARELLI-RAA-1987-CARBOHYD-POLYM-V7-P87
SINGH-V-2006-POLYMER-V47-P254
SUN-LP-2006-POLYMER-V47-P1796
VARMA-RS-1999-GREEN-CHEM-V1-P43
WANG-HJ-2010-BIOMATERIALS-V31-P6589
WANG-XY-2011-CURR-NANOSCI-V7-P183
XU-T-2010-CARBOHYD-POLYM-V81-P931
YUAN-H-2008-J-MACROMOL-SCI-A-V45-P754
ZHU-XM-2009-J-CHEM-SOC-PAKISTAN-V31-P652