UV Spectrophotometry of DNA, RNA, and Proteins
advertisement

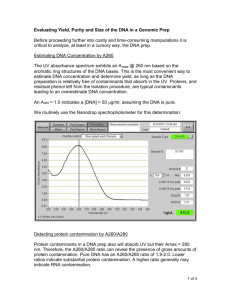
Spectrophotometry Lab # 2 UV Spectrophotometry of DNA, RNA, and Proteins Purpose Molecular biologists routinely work with DNA, RNA, and proteins and have devised some simple, fast spectrophotometric assays for these molecules. The purpose of this exercise is to use the UV absorbance of biological samples to obtain qualitative and quantitative information about those samples. In this spectrophotometry exercise you will: Measure the absorbance at 260 nm to quantify DNA. Calculate A260/A280 ratios to estimate the purity of a DNA preparation. Operate a spectrophotometer in the UV range. Brief Background Review A. The UV Absorbance Spectra of Nucleic Acids and Proteins Most biological molecules do not intrinsically absorb light in the visible range, but they do absorb ultraviolet light. Biologists take advantage of UV absorbance to quickly estimate the concentration and purity of DNA, RNA, and proteins in a sample. It is possible to quantify the amount of DNA in a sample by looking at its absorbance at a wavelength of 260nm or 280nm (in the UV region). The UV method described in this exercise is not highly accurate but it is very widely used since it is easy, quick, and little DNA is required. Proteins have two absorbance peaks in the UV region, one between 215-230 nm, where peptide bonds absorb, and another at about 280 nm due to light absorption by aromatic amino acids (tyrosine, tryptophan and phenylalanine). Certain of the subunits of nucleic acids (purines) have an absorbance maximum slightly below 260 nm while others (pyrimidines) have a maximum slightly above 260 nm. Therefore, although it is common to say that the absorbance peak of nucleic acids is 260 nm, in reality, the absorbance maxima of different fragments of DNA vary somewhat depending on their subunit composition. Figure 1 shows the UV spectra for DNA, RNA, and proteins. Observe that although proteins have little absorbance at 260 nm, both proteins and nucleic acids absorb light at 280 nm. Therefore, if nucleic acids and proteins are mixed in the same sample, their spectra interfere (overlap) with one another. Table 1 below summarizes the UV wavelengths relevant to the measurement of nucleic acids and proteins FIGURE 1 Figure 1. UV ABSORBANCE SPECTRUM FOR DNA AND PROTEINS. A. DNA. b. Protein (BSA). C. The absorbance spectrum for a mixture of DNA and protein. Distinct DNA and protein peaks cannot be resolved. TABLE 1 UV MEASUREMENTS OF DNA, RNA AND PROTEINS WAVELENGTH SIGNIFICANCE COMMENTS 215-230 nm Minimum absorbance for Measurements are generally nucleic acids not performed at this Peptide bonds in proteins wavelength because absorb light commonly used buffers and solvents, such as Tris, also absorb at these wavelengths. 260 nm Nucleic acids have Purines absorbance maximum absorbance maximum is slightly below 260; pyrimidines maximum. is slightly above 260. Purines have a higher molar absorptivity than pyrimidines. Therefore, the absorbance maximum and absorptivity of a segment of DNA depends on its base composition. Proteins have little absorbance at this wavelength. 270 nm Phenol absorbs strongly Phenol may be a contaminant in nucleic acid preparations. 280 nm Aromatic amino acids Nucleic acids also have absorb light some absorbance at this wavelength. 320 nm Neither proteins nor Used for background nucleic acids absorb correction since neither nucleic acids or proteins absorb at this wavelength. B. Concentration Measurements of Nucleic Acids and Proteins in a Sample Determining concentration in a “pure” sample containing only proteins or nucleic acids, involves constructing a standard curve. It is possible to determine the concentration of nucleic acids or proteins based on their absorbance at a wavelength of 260 nm or 280 nm respectively. A calibration curve using standards of known concentration can be constructed. For accurate results, the standard curve should be prepared using the protein of interest or DNA that is similar to that in the sample being measured. The linear range for DNA values is reported to be from about 550 µg/mL. Depending on the protein, UV analysis of proteins at 280 nm has a linear range from about 0.1 - 5 mg/mL. The average absorptivity constants for proteins and nucleic acids lead to the following relationships: If a sample containing pure double-stranded DNA has an absorbance of 1 at 260 nm, then it contains approximately 50 µg/mL of double -stranded DNA. If a sample containing pure single-stranded DNA has an absorbance of 1 at 260 nm, then it contains approximately 33 µg/mL of DNA. If a sample containing pure RNA has an absorbance of 1 at 260 nm, then it contains approximately 40 µg/mL of RNA. Values for proteins vary. A very rough rule is that if a sample containing pure protein has an absorbance of 1 at 280 nm, then it contains approximately 1 mg/mL of protein. For example, 1 mg/mL of bovine serum albumin is reported to have an A280 value of 0.7. Antibodies (which are a type of protein) at a concentration of 1 mg/mL are reported to have an A280 between 1.35 and 1.2. (Values from "Antibodies: A Laboratory Manual", by E. Harlow, and D. Lane, p. 673. Academic Press, New York., 1988.) C. Estimation of the Purity of a Nucleic Acid Preparation It is possible to use UV spectrophotometry to estimate the purity of a solution of nucleic acids. This method involves measuring the absorbance of the solution at two wavelengths, usually 260 nm and 280 nm, and calculating the ratio of the two absorbances: An A260/A280 ratio of 2.0 is characteristic of pure RNA. An A260/A280 of 1.8 is characteristic of pure DNA. A260/A280 ratio of about 0.6 is characteristic of pure protein Therefore, a ratio of 1.8 - 2.0 is desired when purifying nucleic acids. (Note that this method does not actually distinguish DNA and RNA from one another.) A ratio less than 1.7 means there is probably a contaminant in the solution, typically either protein or phenol. Table 2 below summarizes the various UV methods described in this exercise. TABLE 2 APPROXIMATING THE CONCENTRATION AND PURITY OF DNA, RNA OR PROTEIN IN A SAMPLE Concentration of double-stranded DNA ~ (50 µg/mL) X (absorbance at 260 nm) Concentration of single-stranded DNA ~ (33 µg/mL) X (absorbance at 260 nm) Concentration of RNA ~ (40 µg/mL) X (absorbance at 260 nm) Turbidity causes an apparent increase in the absorbance of a sample leading to incorrect readings. To compensate for slight turbidity, a background correction can be used. Proteins and nucleic acids do not absorb at 320 nm. Therefore, if a sample absorbs at 320 nm, the absorbance is due to turbidity. The absorbance at 320 can be subtracted from the readings at 260 nm and 280 nm: Concentration of double-stranded DNA ~ 50 µg/mL (A260 - A320) Concentration of single-stranded DNA ~ 33 µg/mL (A260 - A320) Concentration of RNA ~ 40 µg/mL (A260 - A320) Concentration of protein ~ 1 mg/mL X the absorbance at 280 nm Concentration of protein ~ 15 mg/mL X the absorbance at 215 nm Notes: Values for proteins vary. The rule that 1 mg/mL of protein has an absorbance of 1 is approximate. Tris and other common solvents also absorb light at 215 nm. For this reason, 280 nm is far more commonly used for protein measurements. Purity An A260/A280 ratio of 2.0 is characteristic of pure RNA An A260/A280 of 1.8 is characteristic of pure DNA An A260/A280 of 0.6 is characteristic of pure protein Notes: A ratio of 1.8 - 2.0 is desired when purifying nucleic acids. This method does not actually distinguish DNA and RNA from one another. A ratio of less than 1.7 means there is probably a contaminant in the solution, usually either protein or phenol. Laboratory Activities Use quartz cuvettes in the UV range. Handle them with caution! 1. Measure the A260 and the A280 for each sample. Calculate their A260/A280 ratios. 2. Calculate the concentration of the DNA, RNA, and protein samples based on their A260 values. Record the concentrations of these samples based on what is written on their labels. 3. Place results for the class on the board. Include: a. A260 and the A280 for each sample. b. A260/A280 ratios for the samples. c. Your calculation of the concentrations of DNA, RNA, and protein in the samples based on the A260 values. 4. If your results differ from those of your classmates, try to figure out why. If all students use the same samples, the results should be the same. 5. Try mixing some protein with one of the DNA or RNA samples to see what this does to the absorbance values. SUMMARY OF RESULTS UV SPECTROPHOTOMETRY OF DNA, RNA AND PROTEINS DNA A260 A280 A260/A280 Purity? Concentration RNA A260 A280 A260/A280 Purity? Concentration PROTEIN A260 A280 A260/A280 Purity? Concentration MIXTURE A260 A280 A260/A280 Purity? Concentration Discussion: 1. What is the estimated concentration of DNA, RNA and protein in your samples based on their absorbances at 260 nm? The DNA, RNA, and protein solutions should be labeled with their concentration based on how much the preparer weighed out and dissolved. Generally we make these at 0.1 mg/mL for the DNA and RNA, and 1 mg/mL for the protein. Compare the estimated concentrations based on absorbance to the expected concentration based on the preparation of the samples. Are they the same? If not, assume that the concentrations labeled on the solutions are the correct concentrations. 2. These UV assays are very commonly used by molecular biologists. Why? What information is obtained from these simple assays? 3. What are the advantages and disadvantages of these UV methods? Consider their accuracy, precision, range, ease of use, cost, and any other factors you think are important. Group DNA A260, A280 A260/A280 DNA [] based on A260 RNA A260, A280 A260/A280 RNA Protein [] A260, A280 based on A260 A260/A280 Mix A260, A280 A260/A280 This project is funded by a grant awarded under the President’s Community Based Job Training Grant as implemented by the U.S. Department of Labor’s Employment and Training Administration (CB-15-162-06-60). NCC is an equal opportunity employer and does not discriminate on the following basis: against any individual in the United States, on the basis of race, color, religion, sex, national origin, age disability, political affiliation or belief; and against any beneficiary of programs financially assisted under Title I of the Workforce Investment Act of 1998 (WIA), on the basis of the beneficiary’s citizenship/status as a lawfully admitted immigrant authorized to work in the United States, or his or her participation in any WIA Title I-financially assisted program or activity. “This workforce solution was funded by a grant awarded under the President’s Community-Based Job Training Grants as implemented by the U.S. Department of Labor’s Employment and Training Administration. The solution was created by the grantee and does not necessarily reflect the official position of the U.S. Department of Labor. The Department of Labor makes no guarantees, warranties, or assurances of any kind, express or implied, with respect to such information, including any information on linked sites and including, but not limited to, accuracy of the information or its completeness, timeliness, usefulness, adequacy, continued availability, or ownership. This solution is copyrighted by the institution that created it. Internal use by an organization and/or personal use by an individual for non-commercial purposes is permissible. All other uses require the prior authorization of the copyright owner.”