BIOLOGY : UNIT ONE - Melbourne High School
advertisement

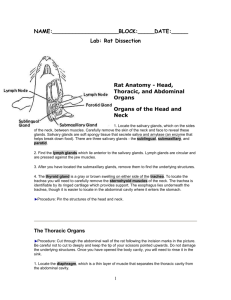
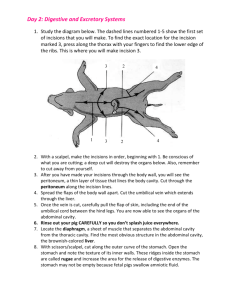
MELBOURNE HIGH SCHOOL UNIT ONE PRACTICAL MANUAL TABLE OF CONTENTS PRACTICAL WORK ...................................................................................................................... 2 Practical Report Format............................................................................................................ 2 Microscopic Examination of Cells .............................................................................................. 3 SAC 2 Surface Area to Volume Ratio ........................................................................................ 5 Mammalian Digestive System .................................................................................................... 6 SAC 3 Food Chemistry ................................................................................................................ 8 THE STANDARD REPORT FORMAT FOR PRACTICAL WORK In Biology, your practical reports have to be written entirely and should follow the accepted format as shown in the sample below. It is important to realise the value an assessor places on the presentation of data in tables, graphs and diagrams, as appropriate. When the experimental exercise is complete, produce your report promptly. In many cases, the Procedure will be a published procedure. In that case, unless you do anything differently, just quote the reference to the published procedure. Do not include the procedure in your report, unless you change any part of it; then only say what you did instead of the published procedure. A SAMPLE PRACTICAL REPORT top of page 12/4/69* left of page INTRODUCTION PURPOSE PROCEDURE RESULTS OR OBSERVATIONS EQUIPMENT DISCUSSION CONCLUSION(S) REFERENCES Studying Pond-life A. Nonymous 5BIA right of page centre of page Write some brief background information relating to the topic for investigation. Write the Aim of the experiment, which should include to the hypothesis being tested. In some cases you must design your own experimental procedure. Otherwise, if you are following a procedure from a laboratory manual or printed notes, merely quote the procedure pages in that manual, or the title of the notes. If you change any part of a published procedure, indicate the change(s), giving enough information for the reader of the report to be able to do the same experiment you did, using the procedure you used. This is where you record all relevant observations, readings, tables of results, etc. Be prepared to record everything you hear, see, smell, taste, feel, etc. Practise constructing tables for the presentation of data. In this section you would also draw diagrams and drawings, for example of microscope slides If you are writing a report of your own experiment, list all materials and equipment you used. Here you comment on how your results and observations compare with your expectations, and how well, or otherwise, they support your hypothesis. You would answer questions about the procedure and could also suggest additional experimentation that might help elucidate your hypothesis. You would also comment on problems encountered, and possible sources of error. Come to some conclusion about the exercise, in terms of how your results related to the stated purpose of the exercise ie. did your results support, or disprove, your hypothesis? Indicate, precisely, the title(s) of any reference(s) used, with the author(s), year of publication and page number(s). * The date to write here is the date you began the practical work, not the date you wrote or submitted the report. BIOLOGY UNIT 1 : Practical Manual p. 2 ANIMAL, PROTOCTIST AND PLANT CELLS This practical is in several parts, and will take at least three periods. To get the most from it will require preparation, perseverance and a readiness to quickly admit failure and to start again, as soon as necessary. PURPOSES 1. To examine several preparations, trying to discover some features of animal and plant cells. 2. To discover similarities and differences between cells of these organisms. 3. To carefully draw cells of as many different kinds as possible. HYPOTHESIS That cells with different functions have different specialised organelles and/or shapes. MATERIALS & EQUIPMENT Microscope; microscope slides & coverslips; small, Pasteur pipette; absorbent paper; razor blade; various tissues, stains & solutions; commerciallyprepared slides; onion; lettuce. PROCEDURE In each case, draw cells large enough as to occupy about ⅙ of a page and indicate next to your cell drawings the microscope magnification you were using when you drew the cell, an estimate of the actual size of the cell (in micrometres [ ]) and, if the cell was stained, the name of the stain used. Always clearly label the cell-type e.g. human cheek cell, stained with methylene blue, mag. 100X (NB this means you used a 10X ocular lens and a 10X objective lens). PART A: USING A MICROSCOPE Follow your teacher’s instructions to learn the parts of a light microscope, and how to use one to rapidly obtain a focused image of a prepared microscope slide. As soon as you learn this part, you will be able to approach the rest of this exercise. QUESTIONS 1. What are two techniques for improving the clarity of the image of transparent material? 2. What are some of the effects on the appearance of the image, of changing from a 10X objective to a 40X objective? 3. What happened to the appearance of the image when you lowered, or raised, the condenser lens system? 4. What is the effect on the image if you move the slide to your left? PART B: PLANT & ANIMAL CELLS 1. Using half an icy-pole stick, scrape some cells from the inside of your cheek. Place them into some 0·9% NaCl on a flat slide. Immediately add a coverslip. Examine the cells microscopically using low-power and, when you locate some cells, switch to high-power and draw as much detail as you can observe. 2. Place a drop of methylene blue stain on one edge of the coverslip and allow it to work its way under the coverslip. Examine the slide and draw a cell affected by the methylene blue. Compare the unstained cells with the stained. 3. Examine a commercially-prepared slide of blood and draw some of the cells with obvious organelles (leukocytes). 4. Now peel a transparently thin strip from an onion (Allium cepa) epidermis and add it to 3 drops of 0.9% NaCl on a clean slide. Add a coverslip and then a drop of iodine solution to one edge of the coverslip. Examine under low-power when the stain has diffused under the coverslip. Draw a few cells in as much detail as possible. 5. Repeat the procedure, but this time do not add the stain. Again, compare the unstained with the stained cells. 6. With the current slide, add two drops of 2.0% NaCl to one edge of the coverslip, and a drop of iodine solution to the other side of the coverslip and allow time for diffusion into the onion tissue, then observe microscopically. Draw some cells in the presence of 2.0% NaCl. QUESTIONS 5. What organelles could you definitely identify in the cells you observed? Say what enabled their definite identification. 6. Did any of the cells have organelles not apparent in the other cells? Say what the organelles were. 7. Explain why you think a nucleus only appears in some of the cells. 8. What was the effect of the stain on the appearance of the tissue/cells? 9. How did the onion cells in 2% NaCl and iodine solution compare visually with the stained onion cells in 0.9% NaCl? What do you think caused any differences in appearance? PART C: PHOTOSYNTHETIC TISSUE CELLS Using a small piece of a crisp lettuce leaf, peel back the lower surface so it snaps partly through; then keep slowly peeling back so you expose a transparent layer of cells. Cut this layer of cells free and place the cells in a couple of drops of water on a flat microscope slide. Add a coverslip and examine the cells microscopically Add a small amount of iodine solution and re-examine. You should see two distinctly-different cell types: one resembling pieces from a jigsaw puzzle (epidermal cells); the other looking like curved, fat sausages facing each other (guard cells)–these ones are always in pairs. A third cell type should be visible near the cleared area–these are very green and much bigger than the other two and are called mesophyll cells. Switch to high-power and draw samples of each of the epidermal cells and guard cells. Pay special attention to the organelles present (or absent) in each cell type. BIOLOGY UNIT 1 : Practical Manual p. 3 QUESTIONS 10. What organelles could you identify in (a) the epidermal cells (b) the guard cells and (c) mesophyll cells? 11. Why are guard cells always in pairs? And what is the name of the structure they surround? 12. What is the function of the structure named in Q. 15? 13. What causes mesophyll cells to look green? 14. What specialised function is performed by mesophyll cells as a result of their organelles? Summarise your findings for the entire exercise in a table relating cell type to organelles found and effect(s) of stains, etc. BIOLOGY UNIT 1 : Practical Manual p. 4 Surface Area to Volume Ratio – Practical Modification This practical is your second SCHOOL-ASSESSED COURSEWORK and requires you to modify the suggested procedure to enable you to investigate the relationship between surface area to volume ratio and the rate of uptake or loss of substances by cells, or tissues such as the absorptive surface of the digestive tract, or the exchange of gases across the alveolar surfaces of the lungs. PURPOSES 1. To investigate diffusion into or out of pieces of agar gel. 2. To modify the procedure to investigate the effect on diffusion rates of different shapes of agar gel pieces. HYPOTHESIS That agar gel pieces of different dimensions will show different rates of diffusion. MATERIALS & EQUIPMENT Dissecting board; razor blade; agar gel made in NaOH and phenolphthalein; 250 mL beaker; vinegar; absorbent paper; plastic ruler. PROCEDURES MODULE 1 1. 2. 3. 4. 5. 6. 7. Take some cubes of agar of different sizes and measure their side lengths. Place the cubes in the beaker and just cover them with vinegar. Record the time. After 10 minutes, examine the cubes by removing them from the vinegar and patting them dry on the absorbent paper. Slice through the centre of each cube. Try to estimate, by measuring the distances across the coloured area of each block, the extent of diffusion in each different cube. Calculate the percentage of the volume of each cube uncoloured after 10 minutes. Complete the table. Cube 3 cm 2 cm 1 cm 0.5 cm surface area (cm2) volume (cm3) SA:V reduced ratio* extent of diffusion (% uncoloured volume) * to calculate the reduced ratio, take the ratio and divide both numbers by the smaller number e.g. if the calculated area was 18 cm2 and the volume 3 cm3, the SA:V ratio would be 18:3; the reduced ratio would then be 18:3/3 = 6:1. QUESTIONS 1. Construct a graph of SA:V against extent of diffusion for each cube. 2. Which has the greatest surface area, a 3 cm side-length cube, or an onion cell? 3. Which has the greatest SA:V? 4. What happens to the SA:V as a cell grows larger? 5. If a mature cell divided by mitosis into two smaller daughter cells, would the daughter cells have the same SA:V as the mother cell? Explain the relationship. 6. What evidence is there that vinegar diffused into the agar? 7. What evidence is there that the NaOH-phenolphthalein diffused out of the cubes? 8. Explain what you think would be a good shape for an organisms that absorbs its nutrient through its external surface. MODULE 2 Write a modified procedure, based on the one you used, to allow investigation of the effects of differences in shape of agar pieces on rate of diffusion. BIOLOGY UNIT 1 : Practical Manual p. 5 Digestive System of a Mammal PURPOSE To investigate the external appearance of the organs of the rat digestive system, and their position in the rat’s body. HYPOTHESIS That the small intestine of a mature rat is 90 cm long. MATERIALS & EQUIPMENT (PER GROUP OF 4): DISSECTING BOARD; COARSE AND FINE SCISSORS; COARSE AND FINE FORCEPS; 4 PIECES OF CORD; 4 THUMB TACKS; WOODEN AND METAL PROBES; RAT (AND ITS BAG); COTTON WOOL Protocol If you want to get maximum benefit from this prac, be prepared to closely follow the procedure below. Work methodically, and read all instructions BEFORE proceeding—you cannot repair any accidental damage. Clean all dissection instruments and wipe them dry between sessions, and at the end of the final session Initial Incision 1. Before removing the rat from its bag snip the top corner from the bag and almost fill it with cold water. Shake the bag to rinse off excess preservative fluid. Pour the water into your sink and repeat the process twice more. Do not destroy the bag (you will need it again), but fully open its top. 2. Lie the rat on the dissecting board on its dorsal surface (back) with its anterior (snout) end facing away from you. Attach cords to each limb and attach the other end of each cord to a thumb tack pushed partially into the board. This will need to be adjusted soon, so don’t push the thumb tacks in too deep. 3. Take a pair of coarse scissors and make a nick through the fur as near as possible to the posterior (genitals) midline. This should not be so deep as to pierce the abdominal cavity. 4. Using a combination of coarse forceps held in the hand you do not use for writing, and coarse scissors in your writing hand, lift the skin with the forceps while cutting with the scissors, making an anterior midline incision as far as the pectoral (shoulder) girdle. Always cut upwards, towards the ceiling, so if you slip, the scissors will come out, rather than going in and damaging tissues below. 5. Now make lateral incisions along each limb; then use the handle of the forceps (or, better still, your index finger) to separate the skin from the body wall, creating flaps of skin. Cut off the skin flaps and place them on some newspaper. Secondary Incision 6. Go back to the point where you made the original skin incision and now make another incision, this time through the body wall. This will be harder, because the body wall is slippery. You might need fine forceps and fine scissors. It is even more important now to cut away from the rat, because now if you slip down, you will damage all manner of organs. 7. Again with coarse forceps and scissors, make another anterior midline incision as far as the pectoral girdle. As before, make lateral incisions along each limb and cut away the body wall flaps, exposing the contents of the abdominal cavity (and, maybe, the thoracic cavity). Dump the skin flaps on the newspaper. Now remove the thumb tacks and adjust the tension on the cords so you separate the limbs as far as possible (if you break the limb joints, it does not matter) and push the thumb tacks back into the board to secure the rat. Abdominal Cavity 8. Draw diagrammatically what you now see, and try to label the organs. Compare what you see with the accompanying diagram, and the dissected rats in the demonstration jars. 9. Using your thumb and middle finger, grip and lift the liver and forcibly remove it from the abdominal cavity. How many lobes does it have? N.B. one organ near the liver and stomach that looks like liver is actually the spleen. 10. You have now exposed the diaphragm at the top of the abdominal cavity (or the floor of the thoracic cavity). Try to lift the stomach and you should find that it’s attached to a tube running through the diaphragm—this is the oesophagus. Cut through the oesophagus to free the rest of the digestive tract, and gently lift the stomach out of the abdominal cavity. Feel the tight ring of muscle that connects the stomach to the duodenum—this is the pyloric sphincter. There is also a dark organ below the stomach—the pancreas, connected to the duodenum by a pancreatic duct. 11. Look carefully at the connective tissue holding the loops of the small intestine in place. This is called mesentery and it allows the small intestine considerable freedom of movement (peristalsis). It also has blood and lymphatic vessels running through it, delivering blood to the intestinal villi and returning the blood to the liver. Sketch a piece of mesentery, then write down a guesstimate of the total length of the small intestine. 12. Now pull on the intestine, breaking the mesentery, until you find a greyish sack attached to the intestine. This is the caecum, the rat equivalent of the human appendix, except that in rats it’s functional—it’s where the rat digests its dietary cellulose. This marks the end of the small, and the beginning of the large intestines. Take a metre ruler and measure the actual length for comparison with your guesstimated length. Then cut the small intestine free. 13. You probably notice that there are several bumps in the large intestine—these are faecal pellets forming; the further they get towards the anus, the drier they get. Check that, by removing some from each end of the large intestine. 14. Pull out the remaining large intestine and cut it free. 15. Place the intestines in your rat-bag, then soak up excess preservative fluid and water with some cotton wool. When you finish the dissection, place all remnants of the rat in your rat-bag, wrap the bag in several layers of newspaper, and deposit the package in the garbage bin. Clean up all dissection instruments and wipe them dry between sessions, and at the end of the final session OBSERVATIONS: What problems did you experience when dissecting the rat? BIOLOGY UNIT 1 : Practical Manual p. 6 How did the contents and its organization appear in the abdominal cavity? List the order of the organs in the digestive system, as you observed them. Draw a labelled diagram of the organs in place after opening the abdominal cavity. DISCUSSION QUESTIONS 1. How do the mouth and teeth compare to those of humans? 2. Can you deduce the type of food rats would generally consume. 3. What proportion of the abdominal region is occupied by the liver? 4. List at least 5 functions of the liver? 5. How much empty space is there in the abdominal cavity? 6. Describe the shape and contents of the stomach. 7. Estimate the length of the small intestine before removing it. My estimate is 8. Remove and measure the small intestine. Its length was cm. cm. 9. Briefly describe the function of each of the following: Mesentery Caecum 10. What happens to the gut contents as it passes into the small intestine and then the large intestine? BIOLOGY UNIT 1 : Practical Manual p. 7 SAC 3: Food Chemistry PURPOSES To become familiar with some standard tests for chemicals present in foods; then to test foods to determine the extent of the presence of those chemicals in various foods. APPARATUS & EQUIPMENT (per pair of students) 2 cavity tiles; 2 cavity slides; 7 semi-micro test tubes; absorbent paper towel; clothes peg; mortar & pestle; spatula; glass rod; Thermoline® water bath; microscope lamp; chemicals kit with samples of glucose solution, starch suspension, cooking oil, sodium chloride solution (NaCl), Benedict's reagent, iodine solution, 1% CuSO4, 10% NaOH, 0.1M AgNO3, wash bottle (distilled water); gelatine, ascorbic acid solution [vitamin C]; 0.1% 2,6 DCPIP. PROCEDURE PART A (Validation Reactions) 1. To establish positive tests for the chemicals glucose, starch, ascorbic acid [vitamin C], protein, lipid (fats or oils), salt and water. Label absorbent paper to correspond to cavity tiles containing known samples of each of the chemicals. 2. To each test tube, cavity tile or cavity slide add a small amount of one of the validation substances (glucose, starch, vitamin C, protein [gelatine], lipid [cooking oil], salt [NaCl] or water) and do each of the tests outlined below. 3. Notice in each case which result is different from all the others – that is the positive test for the appropriate chemical. Benedict's Reagent Test: 1. Add 2 drops of Benedict's reagent to a small amount of the validation substance in a test tube. Make the level up to 1 cm with water from a wash bottle. 2. Place the test tube in the water bath for two minutes. 3. Mark your results sheet to indicate the result. If there was a colour change, say what the colour changed from and to. Iodine Solution Test: Using the cavities in a tile, add 2 drops of iodine solution to the validation substance; in your results table, record any change. DCPIP Test: To materials in the tile cavities, add 1 drop of DCPIP and look for a colour change. Biuret Test: To the material in cavities on a tile, add 3 drops of 1% CuSO4, followed by 1 drop of 10% NaOH and look for a colour change. Paper Test: 1. Add a really small amount of each validation substance to a 1 cm2 piece of paper. 2. Allow the paper to dry in air for at least 20 minutes (meantime, go on with another test). 3. After the paper is dry, record any changed appearance in the paper. Silver Nitrate Test: 1. Using cavity slides, add 2 drops of 0.1M AgNO3, then leave to stand under a microscope lamp for about 3–5 minutes. 2. Record any change. PART B (Food Chemicals Tests) 1. Apply each test to samples of foods and record any positive reactions in Table 2. [It is a good idea not to use foods which are themselves very coloured.]. If the food sample is very dry, or solid, mash a sample with water with a mortar and pestle and test drops of the mash. 2. Record your results by trying to estimate the relative amounts of the chemicals present in any of the foods, e.g. by putting 5+ for a very stronglypositive reaction, but only 1+ for a very weakly-positive reaction. 3. Test a minimum of five foods yourself, and obtain the results for five different foods from someone else, so that your table finally has results for at least ten different foods. DISCUSSION NB In the report for this practical, the major component assessed will be your answers to these discussion questions, so give full, complete answers. 1. 2. 3. 4. Why was it necessary to validate the reagents? What were the positive results, in each case? Explain why you think you had to test water in Part A? Did you (or your partner) find any foods that were very strongly-positive for one chemical only? What food(s) and what chemical tested strongly-positive? 5. Comment on any difficulties you experienced in this practical, and /or any changes you think would have made the practical more meaningful. (This is a major factor in assessment of this Assessment Task). — BIOLOGY UNIT 1 : Practical Manual p. 8