AIEEE Test 6 - meena coaching centre
advertisement
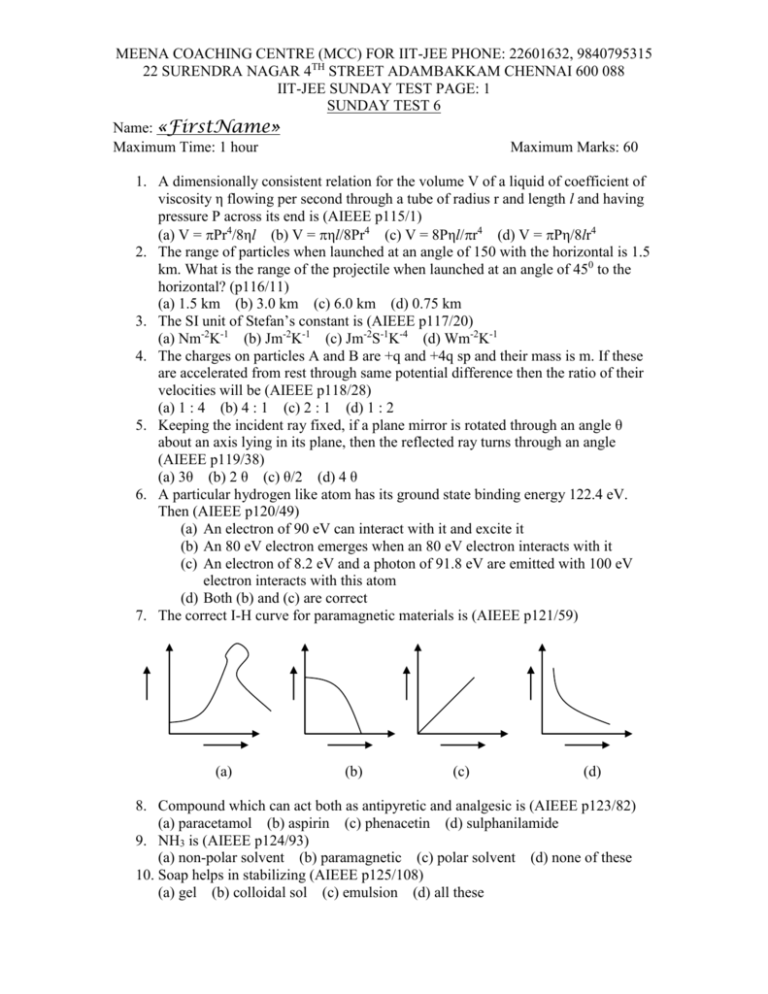
MEENA COACHING CENTRE (MCC) FOR IIT-JEE PHONE: 22601632, 9840795315 22 SURENDRA NAGAR 4TH STREET ADAMBAKKAM CHENNAI 600 088 IIT-JEE SUNDAY TEST PAGE: 1 SUNDAY TEST 6 Name: «FirstName» Maximum Time: 1 hour Maximum Marks: 60 1. A dimensionally consistent relation for the volume V of a liquid of coefficient of viscosity η flowing per second through a tube of radius r and length l and having pressure P across its end is (AIEEE p115/1) (a) V = Pr4/8ηl (b) V = ηl/8Pr4 (c) V = 8Pηl/r4 (d) V = Pη/8lr4 2. The range of particles when launched at an angle of 150 with the horizontal is 1.5 km. What is the range of the projectile when launched at an angle of 450 to the horizontal? (p116/11) (a) 1.5 km (b) 3.0 km (c) 6.0 km (d) 0.75 km 3. The SI unit of Stefan’s constant is (AIEEE p117/20) (a) Nm-2K-1 (b) Jm-2K-1 (c) Jm-2S-1K-4 (d) Wm-2K-1 4. The charges on particles A and B are +q and +4q sp and their mass is m. If these are accelerated from rest through same potential difference then the ratio of their velocities will be (AIEEE p118/28) (a) 1 : 4 (b) 4 : 1 (c) 2 : 1 (d) 1 : 2 5. Keeping the incident ray fixed, if a plane mirror is rotated through an angle θ about an axis lying in its plane, then the reflected ray turns through an angle (AIEEE p119/38) (a) 3θ (b) 2 θ (c) θ/2 (d) 4 θ 6. A particular hydrogen like atom has its ground state binding energy 122.4 eV. Then (AIEEE p120/49) (a) An electron of 90 eV can interact with it and excite it (b) An 80 eV electron emerges when an 80 eV electron interacts with it (c) An electron of 8.2 eV and a photon of 91.8 eV are emitted with 100 eV electron interacts with this atom (d) Both (b) and (c) are correct 7. The correct I-H curve for paramagnetic materials is (AIEEE p121/59) (a) (b) (c) (d) 8. Compound which can act both as antipyretic and analgesic is (AIEEE p123/82) (a) paracetamol (b) aspirin (c) phenacetin (d) sulphanilamide 9. NH3 is (AIEEE p124/93) (a) non-polar solvent (b) paramagnetic (c) polar solvent (d) none of these 10. Soap helps in stabilizing (AIEEE p125/108) (a) gel (b) colloidal sol (c) emulsion (d) all these MEENA COACHING CENTRE (MCC) FOR IIT-JEE PHONE: 22601632, 9840795315 22 SURENDRA NAGAR 4TH STREET ADAMBAKKAM CHENNAI 600 088 IIT-JEE SUNDAY TEST PAGE: 2 11. Phenol is prepared commercially by (AIEEE p126/121) (a) Etard’s process (b) Dow’s process (c) Rasching’s process (d) Deacon’s process 12. Graded ultra filter paper is used in separating (AIEEE p127/138) (a) an emulsion (b) colloidal sol and a crystalloid (c) a colloidal sol from another colloidal sol (d) none of these 13. The colour of [Ti(H2O)6]3+ ion is due to (AIEEE p128/145) (a) d-d transition (b) formation of hydrated ion (c) a complex ion (d) release of larger amount of hydration energy 14. Medicines used to cure mental diseases are (AIEEE p128/149) (a) antibiotics (b) tranquilizers (c) analgesics (d) antipyretics 15. If a, b, c are in G.P., then loga10, logb10, logc10 are in (AIEEE p129/5) (a) A.P. (b) G.P. (c) H.P. (d) none of these 16. The equations: 2x – 3y + 6z = 4, 5x + 7y – 14z = 1, 3x + 2y – 4z = 0 have (AIEEE p130/17) (a) no solution (b) unique solution (c) infinitely many solutions (d) none of these 17. If y = log(1 + sin x), then the value of y4(0) is (AIEEE p131/31) (a) – 1/12 (b) 1/12 (c) –2 (d) 2 18. The area of the region lying between the line x – y + 2 = 0 and the curve x = √y is (AIEEE p132/44) (a) 9/2 (b) 10/3 (c) 9 (d) 3/10 19. Number of solutions of the equation tan x + sec x = 2 cos x lying in the interval [0, 2] is (AIEEE p133/55) (a) 0 (b) 1 (c) 2 (d) 3 20. The pole of the line 9x + 2y = 1 with respect to the ellipse 3x2 + 2y2 = 1 is (AIEEE p134/67) (a) (-1, 3) (b) (-1, -3) (c) (3, -1) (d) (3, 1)









