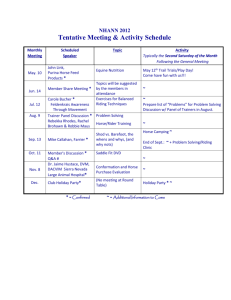
UNDERSTANDING HORSE EVOLUTION
advertisement

HORSE EVOLUTION The impetus for producing this major, gorgeously-illustrated feature was a telephone conversation with a man whom I otherwise enjoy. He’s a cowboy, and grew up on a ranch. He’s a kind fellow who has done a good job with not only a lot of colts, but also his kids, who are likewise very fine people. He’s a Christian – a commitment that I admire – and his children were not only raised in their church, but have gone on to attend Bible College. All of this certainly deserves praise. However, this fellow knows that I’m a paleontologist interested in fossil horses. As we were discussing some of this, at one point in the conversation he raised his voice and said, “I can’t understand it! Belief in evolution is simply stupid!” Well, it’s with any such point of view that I will never be able to agree. In the first place, such an attitude invalidates my experience and beliefs in a way that I would never impose in return – not to mention that it goes against nearly every piece of advice St. Paul ever gave. What could possibly cause an otherwise reasonable man to make such a statement so vehemently to someone he knows it is going to offend? Why -- because the fellow cares about me, of course; he wants my soul to be saved, even at the cost of his own. Why does he think my soul is in danger of damnation? Because he believes that the words he reads in the Bible are literally true – no room for metaphorical interpretations or alternative readings. To this guy, there is one translation of the Bible (I don’t know which one) that is the true and accurate recording of God’s thoughts, desires, and commands for us. Fundamentalism prevents him from being able to reconcile the time-span that geology and paleontology propose for the world, because what mention of that subject there is in Scripture indicates that the world was created less than 5,000 years ago. Fundamentalism has crippled his ability to accept any evidence that would indicate otherwise. But to deny the existence of the (as you are about to see, thousands) of fossilized skeletons of horses and other animals known to science is simply foolish. Worse, to claim as some folks have that they have been “carved out of the rock” in an effort to deceive the public, is an irresponsible lie. And yet, this guy, so schooled in looking for the truth within Scripture, is inclined to believe such lies and distortions. He even gave me a link to a website by a creationist with a Ph.D. degree (in Economics) that spouts even more of them!. I think the news media have also helped to destroy some peoples’ faith in the reliability of science. This even applies to straightforward science reporting. Mis-teaching in the public schools hasn’t helped, either – but the fault for all of this lies squarely at the doorstep of my own profession, which has failed to make an adequate public educational effort. Thus, evolutionist vs. creationist debates are still staged; in Kansas the legislature once again wants to ban the teaching of evolution in public schools; and the people of the United States have elected a string of conservative legislators and Presidents who, responding to strong lobbying by conservative and fundamentalist Christians, have cut Museum funding to the bone. The concept that the public has of what evolution is and how it works simply does not match that which paleontologists hold. In fact, it hasn’t matched for more than a century. When your High School biology teacher taught you that evolution means one animal changing into another animal over a long span of time – and the picture he conveyed was of morphing -- he taught you badly; for there is no known mechanism for “crossfading” among living things. Instead, change through time is iterative by nature – change usually occurs one “click” at a time, like working a lock with a roller dial. When you go to your local Museum and you see an exhibit that shows a single series of horses gradually getting bigger through time – what I call an “inflate-a-horse” exhibit – this is a story that gives the idea that one horse species has morphed into the next. It so far fails to convey the real story that it is almost a crime. This review seeks to convey a more correct idea. Evolution means “change through time”. Charles Darwin said that it occurred as a result of predation, accident, and disease acting upon variability within a population of animals. This was sloganized by Huxley into the “survival of the fittest.” But it needs to be remembered that that what Darwin was proposing was one possible mechanism by which animals in later generations might accumulate enough changes to be recognizable as a species different from the ancestor. Other mechanisms for change through time have also been proposed – the two most famous alternatives to Darwinism coming from Ernst Mayr and Stephen Jay Gould (see the extensive bibliography at the end of this feature for citations to their writings). My personal belief is that there is no incompatibility at all between believing that animals have changed or evolved through time, and that God created the universe. For paleontology says nothing at all about how the universe began; its only interest is in what has unfolded afterward. It is not paleontologists but fundamentalist creationists who arrogantly insist that they know exactly how God has nourished, or how He continues to nourish, this unfolding. In the horse family, change through time is very well documented, based on literally thousands of fossil skeletons that have been unearthed and studied over the past two and a half centuries. Students who wish to understand the subject will want to pay attention to two areas in particular: logical systems for classifying living things, and age determination for the remains of animals that have been found within rock strata. In this feature, I present the information on stratigraphy and age-determination primarily in pictorial form. The rules and procedures of taxonomy (the science of classifying living things) are explained below. The example used throughout is, of course, the horse – so here you will also get a complete classification of the horse along with the actual, technical reasons for that classification. I have also supplied many wonderful photos showing fossil specimens exhibited in museums around the world, so that this issue of “The Inner Horseman” functions as a virtual World Museum Tour. I certainly hope that you find studying this material fun as well as enlightening! Classification Living things are classified on the basis of similarity in structure. This means that you must first notice the structure of whatever living thing you mean to study, noting particularly any features (taxonomists call them “characters”) unique to that creature. Similarities shared by a range of organisms, or features unique to only one or a few of them, can then be tabulated. Characters common to a large number of organisms are said to be primitive. “Primitive” in this sense does not imply crudeness, for all organisms living at all times must have a body plan that works very well in order to stay alive at all. Nor does the term “primitive” necessarily imply that the feature occurs early in time, for in fact most of the characters of most organisms now alive have been inherited unchanged through untold generations, over the span of millennia. Because of this, characters which are unique to a given organism – characters which of necessity must be modifications, great or small, of earlier designs – have special significance in classification, for only such “derived” features can define the boundaries of taxonomic units -- groups of related species. The logical system of classification, called cladistics, makes use of derived features shared by all members of a taxonomic unit to define that unit. Because derived features can only arise from pre-existing designs (those which are comparatively more widespread or more primitive), a cladistic logicdiagram also shows us the most likely order in which the descendants of any given lineage acquired derived features. The science of classification formally began in the mid-18th century with the work of Swedish scientist Carl Linné. Linné (whose name is usually Latinized to Carolus Linnaeus) proposed the system of binomial nomenclature which is still used today. In this naming system, all species receive two names – the first signifying the genus to which the species belongs. When the second term, called the trivial, is paired with the genus name, the two terms taken together uniquely name and identify the species. The example most relevant to our interests would be: Equus – the genus for horses, asses, onagers, quaggas, and zebras. The Latin word “equus” means “weighting all four feet equally”. caballus – the trivial term is a Latin word meaning “nag”. Note that by itself, the trivial term does not identify the horse -- or any other animal. There is, for example, at least one beetle and also one plant whose genus names are followed by the trivial “caballus”. Thus, in order to be sure that we’re discussing the living horse, we need to write: Equus caballus – this binomial term is the scientific name, used in taxonomic classification, that uniquely identifies the horse. Please note the importance of capitalization and italicization. In scientific writing, it is mandatory to capitalize the first letter of the genus name, and to not capitalize the first letter of the trivial. The genus name, which is OK to use alone, or the full binomial when it is used, must be italicized or in some other manner set off from the surrounding text (i.e., boldface or underline are OK too). While these rules are mandatory in any technical journal, good usage demands that they be followed in all publications (so for example, the better-edited newspapers and monthlies such as Equus Magazine scrupulously follow these rules). Naming Species: Importance of the Type Specimen There are also important rules concerning the naming of species. The person who is the first to describe and publish, in an encyclopedia or peer-reviewed journal, any new species gets permanent credit for doing so. The most important rule of taxonomy is that the trivial term is permanently associated with a “type specimen.” Ideally this would be a complete skeleton; practically in horse paleontology it is often a skull, a partial dentition, or even a single tooth. The type specimen must be housed in a Museum of Natural History or other permanent and professionally curated collection, it must bear a unique specimen number, and it must remain accessible in perpetuity for any scientist who wishes to examine it. Once the type specimen has been designated and named, the real work concerning it begins. This consists of observation, discussion, and even controversy among all the paleontologists who have examined it. Because the trivial can never appear, and has no scientific meaning, unless it is assigned to and paired with a genus name, the original namer must assign it to some genus. But that by no means guarantees that’s where it’s going to stay! After examining the type, another scientist may feel justified in reassigning the specimen to a different genus. These re-assignments may go on for years – often because other discoveries, whether in the area of the geology and stratigraphy or as a result of further study of the skeletal material itself, help to put the type specimen into better perspective. There so many examples of this in the paleontological literature that I can only say, “let the student be forewarned”. Here’s a table containing a few examples; boldface type indicates instances where the current expert opinion is that the original namer assigned the species to the correct genus: Original Date & Namer Original Assignment Erwin Hinckley Barbour, 1914 Hypohippus matthewi Present Assignment Megahippus matthewi Edward Drinker Cope, 1873 Anchitherium cuneatum Mesohippus cuneatus E.D. Cope, 1873 Protohippus sejunctus Protohippus sejunctus E.D. Cope, 1879 Anchitherium praestans Kalobatippus praestans E.D. Cope, 1889 Hippotherium isonesum Calippus isonesus E.D. Cope, 1889 Hippotherium sphenodus Griphippus sphenodus E.D. Cope, 1889 Hippotherium retrusum Calippus retrusus E.D. Cope, 1892 Equus simplicidens Equus simplicidens E.D. Cope, 1893 Hippidium interpolatum Pliohippus interpolatus E.D. Cope, 1893 Protohippus lenticularis Nannippus lenticulare E.D. Cope, 1893 Equus eurystylus Neohipparion eurystyle J.W. Gidley, 1903 J.W. Gidley, 1907 Joseph Leidy, 1850 J. Leidy, 1856 Neohipparion whitneyi Merychippus campestris Palaeotherium baridii Hipparion occidentale Neohipparion whitneyi Pliohippus campestris Mesohippus bairdii Cormohipparion occidentale J. Leidy, 1858 J. Leidy, 1869 J. Leidy, 1869 J. Leidy, 1869 Eohippus perditus Protohippus placidus Protohippus supremus Hipparion gratum Othniel C. Marsh, 1874 Pliohippus pernix Pseudhipparion perditus Calippus placidus Pliohippus supremus Griphippus gratus Pliohippus pernix Henry Fairfield Osborn, 1918 Miohippus gidleyi Miohippus gidleyi H.F. Osborn, 1918 Merychippus republicanus Pseudhipparion republicanum H.F. Osborn, 1918 Pliohippus leidyanus Dinohippus leidyanus O.A. Peterson, 1907 Parahippus nebrascensis E.H. Sellards, 1916 Hipparion minor William Berryman Scott, 1893 Anchitherium equinum Parahippus nebrascensis Nannippus minor Hypohippus equinus In all cases, however, even where later research has proven the generic assignment incorrect, the original namer continues in perpetuity to receive credit for naming and describing the type – his name is forever associated with it. So, for example, even though it was Morris Skinner and Bruce MacFadden who did the work and had the insight to reassign the type specimen of Hipparion occidentale to Cormohipparion, the full correct citation for “Cormohipparion occidentale” is Cormohipparion occidentale (Leidy, 1856). The Classification Hierarchy Linnaeus’ original system of classification was hierarchical. A hierarchical classification functions like bowls that stack one inside the other: into the biggest bowl go many organisms, which are themselves bundled into groups. Each of these groups, in turn, functions like a bowl which holds still smaller bowls, and so on until we reach the level of the genus, species, and subspecies. Figs. XXX – XXX graphically illustrate the whole hierarchical classification of the living horse, Equus caballus. Students of the horse need to be familiar with the meaning of such terms as Perissodactyl (vs. Artiodactyl), and Equid vs. Equine (capital “E”) vs. equine (little “e”). All taxonomic terms are defined in terms of structure. In other words, a member of the Order Perissodactyla is identified as such because it possesses certain unique, derived features that are visible on the skeleton. This, at root, is what makes a horse a horse – and not a deer, a goat, a dog, or a primate. Each of the accompanying “learning units” is designed to familiarize you with the particular characteristics that define and characterize each taxonomic unit, whether Kingdom, Phylum, Class, Order, Family, Genus, or species. How Species are Defined The smallest unit recognized by the science of taxonomy is the subspecies. However, where horses are concerned, it is obvious that we often benefit from looking at still smaller units – for example, herds within a subspecies, and even the individual within the herd. The reason that taxonomic classification stops at the level of the subspecies is that this is the level where panmixia occurs. “Panmixia” is a term relating to the genetics of populations. Remember that, in classifying animals, we do not look at their genes; instead, we look at the results of gene action which are manifested as the structural peculiarities of the visible body. Panmixia is the condition which occurs within a population of animals in which every breeding individual has an equal chance of mating with every other individual. When this is the case, any given gene has its best shot at showing up in the maximum number of individuals. “Gene flow” through a panmictic population is free of restrictions or bottlenecks. Subspecies are, by definition, panmictic populations. A species, which may contain one or more than one subspecies, is a group of populations in which any individual is capable of breeding with any other individual to produce viable offspring that can themselves breed to produce viable offspring. In other words, a species is defined by reproduction and gene flow (which in turn creates a certain limited range of skeletal structures within that population). When a species is spread out over a lot of territory – and the horse at one time represented one of the best examples of such – inevitably there will be barriers to reproduction. For example, a mountain range may intervene between the easternmost and westernmost populations of a species. The difficulty in crossing the mountains will make it less likely that individuals from the eastern vs. western populations will meet and interbreed. When there are barriers to panmixia, and when environmental conditions differ from one range to another, populations or herds inhabiting different regions will tend to become adapted to the particular conditions found in that region. This is, of course, not only because they tend to breed only with each other, but also because individuals less physically suited to a given regime of terrain, forage, and climate tend to reproduce less often and produce fewer offspring. They may also be more susceptible to predation, accident, and disease. Over time, the physical type they represent becomes rare and may become altogether extinct. This is how the gene pool of a population becomes characteristic of that population. We detect the range of different gene combinations within a given gene pool through its expression in the physical bodies of individuals. When dealing with the fossil record, we are, of course, lucky to find preserved even one member of any population. It has been estimated that somewhere between 1% and 15% of the skeletons of all the mammals that ever lived were fossilized. Of this small number, only a tiny fraction are actually found, collected, studied, and reported. These statistics are often used as a kind of apology for any mistakes that paleontologists might make in interpreting the fossil record – we have to work from very partial data. However, the same statistic should also be used in reverse -- as a measure of the number and diversity of animals that have lived on Earth. For example, the student may consider the fossil record of horses: in the five largest collections of fossil mammals in North America (the American Museum of Natural History in New York City, the National Museum of Natural History/Smithsonian Institution in Washington D.C., the University of Nebraska at Lincoln, the University of California at Berkeley, and the University of Florida at Gainesville) there are tens of thousands of horse bones and teeth sufficiently complete to permit identification at least to the level of genus. There are also numerous other, smaller collections: The University of Kansas at Lawrence, the University of Michigan at Ann Arbor, the Page LaBrea Tar Pit Museum in Los Angeles, the Los Angeles County Museum of Natural History, and the University of Colorado Museum at Denver, not to mention the National Museum of Mexico in Mexico City, the National Museum of Canada in Ottawa, and the Drumheller Museum in Alberta. If this is the case, it is simply awesome to contmplate the number of animals belonging to the horse lineage that have lived on Earth. A Classification of Horses KINGDOM Animals Animals are multi-cellular animals characterized by the power to move. Although as adults some become rooted by a stalk or pedicle (example: clams, crinoids, anemones), for at least part of their lives they are motile. The cells of animals are thin-walled, lacking stiff cellulose reinforcement. PHYLUM Chordates Animals that have a stiff rod of cartilage extending along the dorsal part of the body. This rod, called a notochord, functions to protect the central nerve cord, which is also located dorsally. In most, the notochord is present only at an early embryonic stage, but in a few (the tunicates) it is present in early life, or (in Amphioxus) it persists throughout life. Chordates possess the full compliment of body systems, i.e. they have a complete digestive tube with a front opening (mouth) and rear opening (anus); central and peripheral nervous systems; segmented muscles; a heart that recirculates blood through a closed circulatory system. There is a brain and sense organs concentrated at the front end of the body, and the body itself is bilaterally symmetrical. The creature breathes by means of a long series of gills, and although there is a mouth opening, there are no jaws. The sense of “hearing” is diffuse, the whole body being sensitive to vibrations in the water; there are no ears. SUBPHYLUM Vertebrates Chordates in which the notochord becomes replaced during embryonic life by a chain of vertebrae which enclose and protect the dorsal nerve cord. Generally, there are additional parts as well – limbs -- to compose an internal skeleton (endo-skeleton). In most vertebrates, the skeleton in the adult creature is bony, but in some specialized forms, such as sharks, the skeleton is cartilagenous. A skull, or at least the lower part of the skull (the palate area and basicranium) is present, and part of the gill series is modified to become jaws that articulate with the skull. A specialized, “concentrated” organ of hearing appears on the side of the head in the area of the jaw joint. The mouth possesses rows of hard teeth. CLASS Mammals Vertebrates which have fur, secrete milk for their young, and whose jaw joint is specifically formed by the articulation of the dentary element of the jaw with the squamosal part of the temporal bone of the skull. This latter is, of course, the criterion by which paleontologists distinguish mammals from near-mammals and non-mammals. Generally speaking, mammals possess teeth of several different shapes – i.e., incisors, canines, premolars and molars; but some mammals lose all teeth or reduce them to vestiges (armadillos, pangolins, aardvarks, sloths), others modify them to fantastic new structures (baleen whales), and still others return to having teeth of uniform morphology (dolphins, killer whales). No matter how modified, however, mammals have only one row of teeth along the margins of the jaws, and they replace only the anterior part of the dental arcade only one time. The teeth develop in, and erupt through the gums out of, sockets called alveoli. Primitively, the limbs of mammals terminate in five digits of about equal length arranged in a fan shape. From this original design many modifications have been tried, including loss of some digits, lengthening or shortening of one or more digits, fusion of digits to form a single stout digit, change from claws to either fingernails or hoofs, or change of the whole limb from a paw into a flipper or a wing. With few exceptions, mammals have exactly 7 cervical vertebrae (whales and dolphins, and also giant ground sloths, have less than 7 vertebrae). Each half of the mandible (the jawbone) in mammals is made up of but one, single element, the dentary bone (in reptiles, amphibians, fish, and birds the mandible is formed either of more than one bony element, or is formed from a different bone than the dentary). The pelvis of mammals is likewise relatively simple, each half being composed of only three elements, the ischium, ilium, and pubis (the pelvis in other vertebrates tends to have additional elements). Importantly, mammals possess a sacrum composed of five or more vertebrae which fuse to make a rod above the pelvis. Birds and frogs also show fusions in this area, but they are far more extensive and serve to prevent flexion of the pelvis on the lumbar vertebrae. In mammals, up-and-down flexion of the spine is a crucial and characteristic element of locomotion, in contrast to the characteristically side-to-side, sinusoidal motions of the vertebral chain in reptiles, amphibians, and fishes (you can tell a fish from a whale or dolphin by the orientation of its tail fins: the fish’s tail fin is vertical, because in order to swim, he “wags his tail” from side to side. Marine mammals, by contrast, have their flukes oriented horizontally, because the main swimming motion is “humping” or up-and-down action of the tail). Every student of the horse should be able to name and know the characteristics of all five of the classes of vertebrates: mammals, birds, reptiles, amphibians, and fishes. By contrasting mammals with the other four, we derive a richer picture of the unique nature of all mammals, and of the horse in particular. ORDER Perissodactyla Herbivorous or omnivorous mammals that retain a caecal digestive system. Perissodactyls have single, obliquely-ridged tibial tarsal bones (astragali), in contrast to the other major order of herbivorous mammals, the Artiodactyla, which have double, parallel-ridged astragali. The term “Perissodactyl” comes from Greek words meaning “digits arranged around (peri-) a central toe (-dactyl)” – and this is indeed the foot-symmetry of all animals belonging to this Order. The central digit, designated by Roman numeral III, is the largest and bears most of the limb’s weight, even when the animal retains three, four, or five toes. This too is in contrast to the Artiodactyla, in which the main part of the weight is shared by closely-appressed or fused digits III and IV. There is a strong tendency to flatten the radius and to closely appress it to the ulna, so as to inhibit or prevent supination of the manus (turning the forefoot inward or upward). In the hind limb, the tibia becomes reduced in size and loses its articulation below with the tarsus. A deep pair of grooves on the distal end of the tibia, which are obliquely oriented to match the ridges on the astragalus, forms a strong, tight, and stable articulation and likewise prevents the hind foot from turning inward. All Perissodactyls have 18 thoracic vertebrae (and thus 18 pairs of ribs), although the number of lumbar vertebrae may be as high as 8. Perissodactyls have bunodont (cusped) or lophodont (ridged) teeth. In the upper dentition, the teeth have the cusps or ridges are so arranged that when the tooth is somewhat worn, they make a shape that looks like the Greek letter “pi”. Worn lower teeth look like the small letter “m”. The tooth formula in Perissodactyls is as follows: in each half of either the upper or lower jaws, there are – 3 incisors 1 canine 4 premolars 3 molars Normally, the tooth formula is written this way: I3C1P4M3. This contrasts with the more advanced Artiodactyls such as sheep, cattle, and camels, which possess no upper incisors. The jaw condyle is lozenge-shaped and transversely oriented. It articulates with a glenoid fossa (formed in the squamous part of the temporal bone of the skull) which is saddleshaped, shallow, and open to the front. The chewing motion is rotatory, with the lower jaw being moved first downward, then laterally, and finally obliquely up-and-across. Food material is actually ground by this last action. Up-and-down chewing action is also possible, but the obliquely-lateral motion is charateristic of all later members of the order. There are a number of important general trends among Perissodactyls: (1) They tend to go for body-size increase as a way to avoid or inhibit predation. They increase not only in height but in bulk – even to extremes, such in Baluchitheres, which are the largest land mammals ever to exist. (2) They tend to lose digits. All of them lose digit I (the thumb) early on; many also lose digit V (the pinkie). Thus, the normal condition in Perissodactyls is to have three or four toes per foot. By contrast, the normal condition in Artiodactyls is to have either four or two toes per foot. (3) As a strategy to make the teeth last longer in eating a tough, abrasive herbaceous diet, they tend to increase the crown-height of the teeth, becoming either hypsodont (highcrowned) or sub-hypsodont. (4) As a further strategy to increase the life of the teeth and thus the potential lifespan of the animal, they tend to increase the complexity of the teeth – which translates in lophodont teeth to increased length of the enamel bands exposed on the tooth crown. (5) They tend to reduce the size of the first premolar tooth (P1 in both upper and lower dentitions), while increasing the size of the molars and the other premolars. There is a strong tendency to form the “cheek teeth” into a battery of uniform or near-uniform size. (6) They tend to develop a long anterior skull with one or more toothless spaces between the cheek battery to the rear and the incisors in front. (7) They tend to have nasal or frontal horns. (8) They almost universally have a short trunk or proboscis, or at least a soft, strong, flexible, semi-prehensile upper lip. All interested students of the horse will want to take every opportunity to look at the mounted skeletons of Perissodactyls which they will find in Museums of Natural History. Throughout time, there have been five major Families of Perissodactyls, to wit: Equids – Hyracotherium, Paleotherium, Parahippus, Griphippus, Astrohippus, Cormohipparion, Neohipparion, Hipparion, Onohippidion, Calippus, Anchitherium, Nannippus, Megahippus and many other fossil genera besides the still-surviving Equus. Tapiroids – The tapir genus Tapirus still survives, but the group was once much more common, including such forms as Homogalax, Helaletes, Hyrachyus, Chasmotherium, Lophiodon, Paleotapirus, and Protapirus. Tapirs are round-backed, moderately heavybodied animals with pronounced retraction of the nasal notch and development of a short proboscis. They live in forests or along the forest edge, eating a mixed diet of leaves, fallen fruit, and insects. Rhinocerotoids – The still-surviving genera Ceratotherium, Dicerorhinus, Diceros, and Rhinoceros plus their fossil relatives, including Hyracodon, Amynodon, Menoceras, Aphelops, Baluchitherium, Teleoceras, Elasmotherium, and many more. Rhinoceroses are moderately to extremely large animals with a thick, tough, largely hairless hide. They characteristically have long, scoop-shaped heads and one or more horns (made of compressed fibers similar to hoof horn) upon their nose. Some diet mostly upon leaves and twigs, while others prefer grass. Chalicotheres – An odd and wholly-extinct group uniquely characterized by long, heavy forelegs with claws. Chalicotheres are the only Perissodactyls that retain and even enhance the ability to supinate the manus. They are thought to have dieted primarily upon leaves and twigs, using their clawed forelimbs, slope-backed build, spout-shaped mouth and long rounded tongue to grasp branches and stip them of leaves. The best-known genus is Moropus. Brontotheres – These animals may be likened to rhinos that have imitated Arnold Schwarzenegger: they are generally larger than rhinos and far more massive through the shoulders and chest. Instead of having one or two conical horns mounted “in line” on their nose, they possess a flat, saddle-shaped horn of bony construction mounted crosswise. Now wholly extinct, the group’s best-known members are Brontops, Brontotherium, Titanotherium, Manteoceras, and Paleosyops. FAMILY Equidae Perissodactyls that tend to retain lightweight bodies, and that tend to a cursorial (running) mode of locomotion. While Hyracotherium, the earliest member of this Family, retains four toes on the forefoot, and while Equus and some other late members of the Family have only one functional digit per foot, the norm is for there to be three functional digits on each limb. This contrasts with the Artiodactyls, in which the norm is for there to be two functional digits per limb. The general tendency among Equids is to lengthen the distal elements of the limbs, i.e. those bones below the carpus and tarsus. They also strengthen and stabilize the distal limb joints: the carpal and tarsal bones articulate closely together, and are formed as block-like rather than rounded elements. The carpal bones are arranged into proximal and distal rows. The distal row tightly articulates with, and is firmly tied by ligaments to, the top of the metacarpal bones below, so that when the carpus folds only two joints open: that between the proximal carpal row and the distal end of the radius and ulna, and that between the two carpal rows. Equids reduce the number of lumbar vertebrae to six, while enlarging the sacrum and retaining 18 thoracic and 7 cervical vertebrae. Dentally, Equids are unique among herbivores in not only possessing, but uniformly enlarging the upper incisors. They typically possess a strong set of incisors not only in the upper but in the lower jaws, and are thus the only mammals whose dental functioning depends upon a “three point” balance, i.e. when the jaws are closed and centered, pressure is shared by the incisors, cheek teeth, and the jaw joint. SUBFAMILY Equinae (Please note that the taxonomist’s use of the word “Equine” – capital “E” – differs from the way your veterinarian uses the word “equine” – little “e”. In veterinary parlance, “equine” means any of the living horse-like animals, i.e. not only domestic horses but Przewalski horses, zebras, onagers, or asses. These are also Equines – but the Equinae also includes a large number of extinct forms known from the fossil record). Equines are Equids adapted for eating grass. Thus, they have hypsodont teeth with cementum, a bone-like material, to support and strengthen each tooth. They have stout, wedge-shaped skulls in which both the maxilla and dentary are deep to accommodate the tall-crowned teeth. The superior teeth of Equines have two fossettes. Each fossette is filled, or largely filled, with cementum. Except for the first premolar, which is greatly reduced in size, the premolar and molar teeth of equines are large, square in cross-sectional shape, and tightly appressed to each other to form “cheek batteries” for the efficient grinding of grass blades. There is a strong tendency among equines to retract the nasal notch, i.e. to “undercut” the nasal bone, even to an extreme degree (vis., Hippidium and Onohippidion). In such forms, deep pits or “facial fossae” tend to be present on the face, the complex as a whole indicating the presence of a small trunk or a strongly prehensile upper lip longer than that in the living horse. Where there is little or no retraction of the nasal notch (Neohipparion, Nannippus), the face will be smooth, lacking facial fossae. Forms that have smooth faces tend to have the most complex and most highly hypsodont teeth. Equines are universally unguligrade, having hoofs rather than claws and never locomoting with the hock, carpus, or “ankles” (the joint between the distal end of the cannon bone and the pasterns) touching the ground. Generally speaking, they are are tall and have proportionally long distal limb elements. They are adapted for straight-line flight over firm substrates in open terrain. Equines characteristically develop joints between the “wings” of the sacrum and the transverse processes of the last lumbar vertebra. They also have articulations between the transverse processes of the last several lumbar vertebrae. Moreover, the joints between the accessory articular processes in the lumbar vertebrae of equines are verticallyoriented, and they are shaped to articulate like dovetail joints. These “inter-transverse” and “dovetail” articulations almost totally inhibit rotation and lateral flexion among the lumbar and lumbo-sacral joints, while promoting up-and-down coiling of the lumbosacral joint and loin-span. This is in sharp contrast to Artiodactyls, which retain long, relatively loosely-articulated lumbars which permit twisting and a greater degree of lateral flexion. INFRAFAMILY Protohippini Protohippine equines are distinguished by possessing high-crowned teeth that are nevertheless comparatively simple in structure (the genus Equus has the most complex teeth within the Protohippine lineage). An important distinguishing characteristic of the Protohippine dentition which is easy to see is that the protocone loop of enamel is joined to the hypoloph, not set off (as it is in Hipparionines) as a separate circlet. Two kinds of skull and skeletal structure may be found within the Protohippini: one is the “normal” or mesomorphic build we associate with the genus Equus. These animals have flat or slightly-rounded backs, nasal notches only moderately retracted, moderately prehensile upper lips, and smooth faces. The other type re-echoes the “chalicomorph” or “okapi-like” body design in having forelimbs longer than hind limbs, a sloping back, deeply-retracted nasal notch, and deep facial fossae for the attachment of the muscles to move a long, strongly prehensile upper lip or short trunk. Pliohippus is an outstanding example of this, and study of Pliohippus should remind students never to fall back into the old 19th-century “evolutionary progression”, proposed by O.C. Marsh, that supposedly led from Protohippus through Pliohippus to Equus. Equus is the descendant of Protohippus and Dinohippus, both flat-backed and smooth-faced genera, not Pliohippus. Not surprisingly, to go along with the two different body-styles found within the Protohippini, there are two sorts of dentition. Equus and earlier members of its direct lineage have relatively straight teeth, with the angles of the “tables” or occlusal surfaces of the cheek batteries set at from 7 to 10 degrees of slope. Pliohippus and other “okapilike” forms have upper teeth with a lot of curve to them, and thus table angles ranging from 10 to 25 degrees of slope. These teeth have exceptionally heavy outer styles and buttresses, and very simple lower teeth; such a design is less for dealing with grass than with “chop”, i.e. twigs, leaves, and bark rather than grass. The supposition is that Pliohippus and similar forms were eating a diet similar to a deer’s. The other infrafamiliar taxon is the Hipparionini. Students of the horse should take every opportunity, by visiting Museums of Natural History, to familiarize themselves with these animals as well. Hipparionines are typically small, narrow-bodied, lightweight, agile and dainty. While Protohippines tend to be large and heavy, Hipparionines are relatively small, some even becoming dwarfs no larger than the African dik-dik. All Hipparionines have extremely hypsodont teeth with highly complex structure, good for processing dry forage. These animals were, evidently, filling the ecological niche now exclusively occupied by antelopes. GENUS Equus Members of the genus Equus have but one functional toe per foot. It should be noted that Equus is not the only horse genus to become monodactyl; for example, there is a species of Neohipparion from the Ashfall Quarry in northern Nebraska that achieved this condition long before either Equus (or its direct ancestor Dinohippus) came into existence. Nevertheless, monodactyly is a derived character shared by all members of Equus, and is thus a diagnostic feature of the genus. As previously stated, Equus has highly hypsodont teeth which have complex, more wrinkled enamel structure than other members of its lineage. Once again, however, Equus does not have the most high-crowned teeth known among Equids: Hipparionines have teeth which are equally or more high-crowned than Equus, and there is even one species of Nannippus known from Florida which actually develops hypselodont teeth in which the roots never close and the tooth is “ever-growing”. Nor does Equus have the most complex enamel structure; that honor belongs to Cormohipparion, Neohipparion, and some of the European species of Hipparion. Students of the horse should, by visiting a Museum of Natural History, study the remains of all the fossil horses. There are many more genera than the old “hippuses” ladder-ofdescent litany tends to include, and, although I repeatedly admonish students to visit their local Museum, I forewarn you that many of the exhibits you see there are likely to be sadly out of date (this is because since the Carter Administration, and much more seriously since the Reagan Administration, federal funding for Museums and for all forms of so-called “esoteric” research has been cut to the bone. This is not the only reason, but it is an important one, why the author herself does not work in a Museum). So, when you visit the Museum you may see a “horse evolution exhibit” that still features the hackneyed and incorrect series that typically starts with “Eohippus” (Hyracotherium), and then proceeds through Orohippus, Mesohippus, Miohippus, Merychippus, and Pliohippus, finally to terminate with Equus. But you may know that paleontologists are currently aware also of the following genera (in alphabetical order): Anchitherium Archaeohippus Astrohippus Calippus Cormohipparion Dinohippus Epihippus Griphippus Hipparion Hippidium Hypohippus Kalobatippus Megahippus Nannippus Neohipparion Onohippidion Palaeotherium Parahippus Pseudhipparion SPECIES Equus caballus Equus caballus is the heaviest and one of the tallest species in its genus, and it also happens to be one of the largest and heaviest of all equids. It has the broadest and deepest skull and the stoutest limbs. The upper cheek teeth are large, usually with long, bipartate protocones that look somewhat like a Dutch shoe (Fig. XXX). In general, the enamel plications visible upon the working surfaces of the upper teeth are quite complex; a pli caballin is typically present. The inferior cheek teeth show a “U”-shaped enamel reentrant (the ectoflexid). The incisor teeth are large and all of them, even the lateral incisors, possess both infundibulae (“cups”) and dental marks (“stars”), which can both, at a certain stage of wear, simultaneously be present in a given tooth. Although the incisor row is broad, it is not as broad as in some fossil genera, e.g. for example Calippus, nicknamed by paleontologists the “lawn mower horse” (Fig. XXX). There is little inflation of the frontal sinuses in most animals, so that they have straight faces and flat foreheads. One very useful characteristic (if one has a complete or nearlycomplete skull to identify) is that the temporal bone of the skull forms a broad triangle behind the ear region in this species, broadly separating the postglenoid and paramastoid processes. This is a reflection of the fact that the occiput slopes backwards rather than being tucked under. The practical result is that you can tell the skull of a horse from that of an ass, mule, zebra, or onager by standing it up on end. The horse skull will balance; skulls of the mule and of the other species will fall forward. Living Species of Equus Students of the horse should help themselves to put the horse into context by comparing it with the other eight living (or recently extinct) species in the genus Equus. You should realize at this point how closely related to the horse these animals are; the tragedy is that all of them are now on the brink of extinction (and the horse itself has been, since 1947, extinct in the wild). Since most of these very interesting animals can be seen in zoos, you should familiarize yourself with what they look like: Equus zebra – the “true zebra”. There are two subspecies, the Hartmann’s zebra (E. zebra hartmanni), and the Mountain zebra (E. zebra zebra). This species is distinguished by its donkey-like body size and form, by a “gridiron” pattern of stripes over the top of the croup, and by the fact that there is a flap of skin on the underside of the throat. Equus burchelli – the Bontequagga, Plains zebra, or common zebra. This animal has a pony-like body form and an undulating facial profile. It completely lacks infundibulae in the lateral incisors. Its stripes are broad, although some forms may have thinner stripes lying in the white zones between broader black stripes. The belly is white or creamcolored and unstriped; sometimes the light tone extends far up on the body, while there are also melanistic individuals whose white color is confined to rows of white dots. Many zoos distinguish different forms based on the exact pattern of striping, e.g., , but in actuality they are all members of a single species. This is the commonest zebra to be seen in zoos, may often also be seen in the circus, and is the most populous in the wild (although still an endangered species). Equus grevyi – the narrow-striped zebra, Abyssinian zebra, or Grevy’s zebra. This animal is the oldest of all African equids, its fossil record there extending back at least five million years. It is tall, sometimes reaching over 15 hands in height, and has fairly prominent withers. The skull is long and narrow, and the facial profile may be either straight or slightly undulating. It has the simplest enamel pattern on its teeth of any Equus; in the lower teeth, the “U”-shaped ectoflexid re-entrant reaches all the way across the teeth. Equus onager – the Indian and west-Asian Onager, Onaigre, Khur, or Kulan. One of three so-called “hemione” (meaning “half like an ass”) species, the Onager is an orangecolored animal with a buffy to white underbelly. It has ears longer than those of a horse but shorter than those of an ass; they are pointed like those of the horse and ass, rather than being rounded like those of zebras. Onagers and other half-asses are distinguished by having proportionally long, narrow cannon bones, short wedge-shaped heads, and lower cheek teeth in which the ectoflexid re-entrant does not penetrate all the way across the tooth. Equus hemionus – the Dzhungarian (Chinese and Mongolian) hemione, Chiggetai or Dziggetai. This is the tallest and most gracile of the half-asses, and also the rarest to be seen in Western zoos. Its coat is a dusty-gray color and the back and belly contrast less than in the onager and kiang. Equus kiang – the Tibetan Kiang. This is hemione has the shortest, stoutest limbs and overall the stoutest build. Its pelage is a rich blackish-brown, standing in sharp contrast to the nearly-white fur of the underbelly and the inner flanks. Equus asinus – the ass. Besides the domestic donkey, there were once wild donkeys in the Arabian Peninsula, and until the recent series of civil wars in Somalia, there was a population surviving there also. Equus quagga – the South African Quagga. This animal became extinct in 1904, having once roamed the plains of South Africa in countless numbers. It was exterminated by the Afrikaaners as the buffalo were nearly exterminated by Anglo-Americans. The animal had a body form similar to that of a Burchell’s zebra, but was typically striped only upon the legs and over the top of the croup. My own analysis of quagga skulls – there are only five known to science – indicates that these animals deserve to be recognized as a separate species more similar to a horse than to a zebra. However, amino-acid assay tests performed on preserved quagga hides indicate that quaggas are zebras. Until I see the results from a DNA test performed with modern techniques, I am remain inclined to believe what the morphological analysis tells me, for unlike any zebra, the lateral incisors of quaggas possess full infundibulae – just like a horse. Along with the horse, the onager and the ass were both brought into domestication at least 4,000 years ago. In addition, all three species of zebra have in historical times been captured, tamed, ridden, driven, and trained for performance in the circus. Mules as normally bred in Europe and the Americas are the product of a human-planned cross between a jack ass and a mare (i.e. E. caballus X E. asinus), but there are other ways to get mules: African farmers frequently make use of zebra semen to get mules from mares, because they have much greater immunity to African parasites and stand the climate better (horses never occurred in sub-Sarharan Africa until people began bringing them there in the 16th century). Zebra-horse mules are called by various names such as “zebrules” or “zorses”. Fossil Species of Equus As if the abundance and diversity of living members of the genus Equus were not enough, we also have an extensive record of this animal in the Northern Hemisphere beginning with its origin at the end of the Tertiary Period. The genus immediately ancestral to Equus is Dinohippus. This animal is similar to Equus in every respect – so much so that I cannot find any characteristics to distinguish them. I continue to use the term Dinohippus, therefore, merely as a way of distinguishing certain species that lived in North America during the Hemphillian and Blancan Land Mammal Ages, between 7 and 5.5 million years ago. (This is a good example of the sometimes uneasy marriage between the logic trees generated by cladistic analysis and the older method of drawing “phylogenetic trees” showing the descent of a species through time. Properly, some day, it will probably be necessary to re-classify Dinohippus by making it a sub-genus, and then we will write, for the best-known species in the genus, “Equus (Dinohippus) interpolatus”). Dinohippus gave rise to a species, Equus francescana, that migrated from California across the Bering Land Bridge during the earliest phase of the Pleistocene when sea-level was lowered. Fossils of this species found in the Old World are called Equus stenonis, and in this form it spread throughout that continent and into Europe and Africa. In Europe, it gave rise to such species as Equus sussenbornensis, the ancestor of Equus mosbachensis, and Equus bressanus – the latter being the probable ancestor of asses. In western Asia and north Africa, Equus stenonis appears to have given rise to Equus hydruntinus, another ass-like form, and Equus teilhardi, which is probably an onager. In Africa, it ultimately gave rise to Equus grevyi. In North America, Equus francescana gave rise to Equus simplicidens. Equus francisi, an onager-like species, also appears very early; it may be derived from E. francescana or from the back-migration of a Eurasian form. Likewise, E. simplicidens is a form which in many respects parallels Equus grevyi, having a very long head, big body, stout legs, and a relatively simple dental pattern. The “stilt-legged” Equus francisi, on the other hand, is not only longer in the shanks but smaller and lighter-bodied, with a more complex enamel pattern in the teeth. From Equus francescana, and also possibly from forms back-migrating from the Old World, there came an enormous proliferation of North American species of the genus Equus. There really are quite a few of these, but those of you wishing to read the techical literature should be forewarned that paleontologists eager to make their mark in this fertile field of investigation have, over the past two centuries, almost hopelessly muddled matters by naming hundreds of species, most of which must be regarded, for a variety of reasons, as invalid “duplicates”. I therefore discuss here only those species that I think are really unique and valid. Bloodlines of the genus Equus continue through three Land Mammal Ages: the Blancan, which is the tail-end of the Tertiary; the Irvingtonian, which is the earlier part of the Pleistocene; and the Rancholabrean, which is the later part. In each of these time periods, Equus species can be classified into either “stout-legged” (horse or zebra-like) or “stiltlegged” (onager-like) forms. (Ass-like forms are difficult to distinguish, and apparently rare, in the fossil record from the New World): Blancan Stout-legged forms: long head, relatively simple teeth, large size Equus francescana Equus simplicidens (old names for this form that you might still see in a museum exhibit are Plesippus, Equus shoshonensis). This form is commonly exhibited because a large number of skeletons have been recovered from the famous Hagerman Quarry near Hagerman, Idaho. There is a complete skeleton of this species on exhibit in a small Museum run by the National Park Service in Hagerman, and at some seasons you can also take a bus tour to view the actual quarry itself. Equus simplicidens skeletons are also on exhibit at the U.S. National Museum of Natural History/Smithsonian Institution in Washington, D.C.). Stilt-legged form: shorter head with broader snout, relatively complex tooth structure, smaller size Equus francisi (first appearance) Typically Irvingtonian (though some range from late Blancan up into the Rancholabrean): Stout-legged forms: Equus niobrarensis Equus hatcheri Equus scotti (See a complete skeleton of this form on exhibit at the American Museum of Natural History in New York City). Stilt-legged forms: Equus francisi (later occurrences) Equus arellanoi Equus calobatus Equus zoytalis Typically Rancholabrean (though some originate in the Irvingtonian): Stout-legged forms: Equus caballus Equus occidentalis (See a complete skeleton and many skulls of this form on exhibit at the Page La Brea Tar Pit Museum in Los Angeles, California, and at the U.S. National Museum of Natural History/Smithsonian Institution in Washington, D.C.) Equus amerhippus Equus excelsus (See a complete skeleton of this form on exhibit at the Nebraska State Museum of Natural History in Morrill Hall on the campus of the University of Nebraska at Lincoln). Stilt-legged forms: Equus altidens Equus quinni Equus conversidens (See a complete skeleton of this form on exhibit at the American Museum of Natural History in New York City). Subspecies of Equus caballus In a recent publication, my co-author Robert Hoffmann and I recognize seven subspecies within the species Equus caballus. (This paper is posted in its entirety at the Equine Studies Institute website, www.equinestudies.org, under “Knowledge Base”). The first item to take care of under this heading are all the synonyms for Equus caballus – “duplicate names” proposed, as previously mentioned, by paleontologists eager to name horse types. Here is a list of published synonyms of Equus caballus with their namer and the publication date; you can see that this tendency for the proliferation of names goes right back almost to the beginning of the science: Equus ferus (Boddaert, 1785) Equus sylvestris (Brincken, 1828) Equus przewalskii (Polyakov, 1881) Equus mosbachensis (Reichenau, 1903) Equus hagenbecki (Matschie, 1903) Equus gmelini (Antonius, 1912) Equus laurentius (Hay, 1913) Equus niobrarensis alaskae (Hay, 1913) Equus abeli (Antonius, 1914) Equus mexicanus (Hibbard, 1957) Equus midlandensis (Quinn, 1957) Equus algericus (Bagtache, Hadjonis and Eisenmann, 1984) Dr. Hoffmann and I recognize seven subspecies within the species. These are forms that we distinguish on the basis of their morphology as well as their mapped geographic occurrence. The seven valid subspecies are: Equus caballus alaskae – the Lamut or Alaskan wild horse. Became extinct about 10,000 years ago, near the end of the Pleistocene. Once ranged in northeastern Asia and across the Bering Land Bridge into Alaska. Equus caballus mexicanus – the American peri-glacial horse. Became extinct at the end of the Pleistocene. Once ranged from the glacial margin west of the Mississippi, south into northern Mexico. Neither of these forms were ever domesticated, as they were already extinct by the time horse domestication began. Equus caballus przewalskii – the Przewalski horse or Mongolian wild horse. Its last known range was a narrow strip in the Gobi Desert, but it once ranged broadly across Russia and Siberia from the Ural Mountains to northeastern Asia. You may frequently see this animal in zoos. It became extinct in the wild in 1947, but survives in zoos and preserves today, all living animals being the descendants of only 13 wild-caught ancestors. The Przewalski horse has contributed in a minor way to a restricted number of Asian breeds in Mongolia, northern China, and eastern Tibet. It is, as I have explained in both the “Mammalian Species” and “Origin of the Mustang” papers posted in our Knowledge Base, most definitely NOT the ancestor of the domestic horse. Equus caballus ferus – the Tarpan. This animal became extinct in 1913, a herd being kept up until that time in the Bialovesh Forest Preserve in Poland. Once ranged from eastern Poland east to the Ural Mountains. This form was the first wild horse to be domesticated. Its characteristics are preserved within several Russian, eastern European, and west Asian breeds including the Konik, Hucul, and Akhal-Teke and related Turkmenian breeds. Through these in turn, Tarpan characteristics have been incorporated into the Thoroughbred. Equus caballus pumpelli – the Afro-Turkic horse. This is the immediate ancestor of our “Oriental” breeds, including the Arabian, Old Hittite, Persian, Bashkir, Lokai, Marwari, and Barb. Equus caballus mosbachensis – the Central European horse. The most ancient and primitive form of Equus caballus, the Mosbach horse is the immediate ancestor of the Warmblood breeds, including the Latvian, Grönigen, Friesian, Cleveland Bay, and all the German Warmbloods and their derivatives now bred in Sweden, Denmark, and the Low Countries. Equus caballus caballus – the West European horse. This is the cold-and-wet adapted subspecies, the form that has the stoutest, most rounded body, the shortest ears, the broadest and deepest head, the shortest legs, the broadest feet, and the thickest fur, mane, and tail. It is native to those parts of Europe that lie west of the Rhine and Rhone rivers, from Norway and Sweden in the north through the Low Countries, the British Isles, western France, and Spain. E. c. caballus is the immediate ancestor of all the draft breeds, such as the Belgian, Clydesdale, Brabant, and Suffolk Punch and their derivatives now bred in other countries, such as Russia and the Americas. It is also the immediate ancestor of all the pony breeds, large and small, including the Fjord, Shetland, Exmoor, Dartmoor, Mehrens, Galician and Asturian. Ponies and draft horses all derive from a single ancestry, the overall size of a given form being remarkably concordant with the area of land upon which the form developed, i.e. Continental forms are the largest, those occurring on the main British island next biggest, and those occurring on offshore islands like Man, the Shetlands, and the Orkneys being the smallest. INTRODUCTION TO THE FAMILY-LEVEL REVIEW The following text is reprinted almost exactly as it originally appeared in the Elsevier World Encyclopedia of Animal Science, Volume C-7, “Horse Breeding and Management”. It is a review, written in semi-technical language, of the horse family. It includes a cladogram with characters. The interested student will want to carefully study this logic-diagram, noting that only ONE “shared-derived” character is sufficient to define any given clade (though more such characters are always desirable). This paper contains many citations – that is the custom in peer-reviewed scientific reporting. All of the citations are listed in the bibliography which follows. In addition to works cited in “The Evolution of the Horse Family”, the bibliography also lists a large number of other papers with annotations as to which fossil species may have been discussed or illustrated in those papers. Many of these works are very old – you will note the long series by the prolific 19th-century American paleontologists Joseph Leidy and Edward Drinker Cope. These men lived at a time when many fossil horse remains first came to light. They named and were the first to describe most of the species. That most of these species have now been placed in genera other than the one originally assigned does not, by the rules of taxonomy already explained, invalidate the type or the species name. It certainly does not invalidate the work of these brilliant and dedicated men, either. There continues to be large interest in research on the horse family. The genus Equus actually has its own “fan club” (a technical interest-group) within the Friends of the Pleistocene of the Society of Vertebrate Paleontology. I have been welcomed as a member of this group and for that I am grateful. Thanks to the continuing, diligent efforts of paleontologists -- professionals and amateur fossil-hunters alike – new discoveries continue to be made which add to the thousands of skeletons already known. From these comes our only hope of correctly recording and interpreting diversity, environmental adaptation, and change through time in the horse family. THE TECHNICAL READING LIST The following is a list of publications from which information used to compile this article comes. If you want to pursue the technical literature, you should print this bibliography out and take it to the library with you, where you will find that it saves you a great deal of time. The ordinary public library probably will not have or be able to locate most of these listings for you; you will have to access either a University library or a library within a large Museum of Natural History, such as the American Museum in New York City, the British Museum in London, the National Museum of Canada in Toronto, the National Museum of Mexico in Mexico City, or the U.S. National Museum/Smithsonian Institution in Washington, D.C. Online Internet listings of the U.S. National Library may also be of assistance; go to http:/catalog.loc.gov or do a Google search for “Library of Congress catalog”. Abusch-Siewert, S. 1983. Gebissmorphologische Untersuchungen an eurasiatischen Anchitherien (Equidae, Mammalia) unter besonder Berücksichtigung der Fundstelle Sandelzhausen. Courir Forschungsinstitut Senkenberg 62:1-361. Agassiz, L. and A. Gould. 1851. Principles of Zoology. Gould and Lincoln Publishers, London. Akersten, W.A., H.A. Lowenstam, and A. Walker. 1984. “Pigmentation” of soricine teeth: composition, ultrastructure, and function, in Am. Soc. Mammal. 64th Ann. Meeting, Abstracts. Humboldt State University, Arcata, California, no. 153, p. 40. Alberdi, M.T., L. Caloi, and M.R. Palombro. 1992. Pleistocene equids from Western Europe: their biostratigraphy and palaeoecological characteristics, in F. Spitz et al., eds., Ongulés/Ungulates 91 (Toulouse, France). Société Francaise pour l’Etude de la Protection des Mammifères, Paris; and Institut de Recherche sur les Grands Mammifères, Toulouse, pp. 31-35. Albritton, C.C. 1980. The Abyss of Time. Freeman, Cooper, and Co., San Francisco. Alexander, R. N. 1988. Elastic Mechanisms in Animal Movement. Cambridge University Press, New York, 141 pp. Alexander, R.N. 1989. On the synchronization of breathing with running in wallabies (Macropus spp.) and horses (Equus caballus). Jour. Zool. London, 218:69-85. Alexander, R.N., N.J. Dimery, and R.F. Kerr. 1985. Elastic structures in the back and their role in galloping in some mammals. Jour. Zool. London, 207:467-482. Allen, J.A. 1878. The geographical distribution of mammals. U.S. Geological Surv. 4:313-376. Ambrose, S.H., and M.J. DeNiro. 1989. Climate and habitat reconstruction using stable carbon and nitrogen isotope ratios of collagen in prehistoric herbivore teeth from Kenya. Quaternary Research, 31:407-422. Anderson, Elaine. 1984. Who’s who in the Pleistocene: a mammalian bestiary, in P.S. Martin and R.G. Klein, eds., Quaternary Extinctions: A Prehistoric Revolution. The University of Arizona Press, Tucson, pp. 40-89. Antonius, O. 1912. Was ist der "Tarpan"? Naturw. Wochenschr. 2(11):27. Antonius, O. 1914. Equus abeli nov. spec. Beitr. Pal. Geol. Ost.-Ung. Or. 26:1913-1915. Antonius, O. 1919. Untersuchung uber den phylogenetischen Zusammenhang zwischen Hipparion und Equus. Zeitsch. Fur induktive Abstammungs-und Vererbungslehre 20(4):273-295. Antonius, O. 1929. Streitfragen zur Phylogenie der Equiden. Verh. Zool.-Bot. Ges. Wien (1928). Arambourg, C. and J. Opieveteau. 1929. Les vertébrés du Pontian de Salonique. Ann. Paléont. 18:59-138. Archibald, J.D., P.D. Gingerich, E.H. Lindsay, W.A. Clemens, D.W. Krause, and K.D. Rose. 1987. First North American Land Mammal Ages of the Cenozoic Era, in M.O. Woodburne, ed., Cenozoic Mammals of North America: Geochronology and Biostratigraphy. The University of California Press, Berkeley, pp. 24-76. Arroyo-Cabrales, J., E. Johnson, and R.W. Ralph. 1993. New excavations at San Josecito Cave, Nuevo Leon, Mexico. Current Res. in the Pleistocene, 10:91-94. Axelrod, D.I. and H.P. Bailey. 1969. Paleotemperature analysis of Tertiary floras. Palaeogeography, Palaeoclimatology, Palaeoecology, 6:163-195. Ayala, F.J. 1988. Can “progress” be defined as a biological concept? In M.H. Nitecki, ed., Evoljutionary Progress. The University of Chicago Press, Chicago, pp. 75-96. Azzaroli, A. 1982. On Villafranchian Palaeontographica Italica, new ser, 72:74-97. Palaearctic Equus and their allies. Azzaroli, A. 1990. The genus Equus in Europe, in E.H. Linsay, V. Fahlbusch, and P. Mein, eds., European Neogene Mammal Chronology, Plenum Press, New York, pp. 339356. Azzaroli, A. 1992. Ascent and decline of monodactyl equids: a case for prehistoric overkill. Annales Zoologica Fennici, 28:151-163. Azzaroli, A. 1995. A synopsis of the Quaternary species of Equus in North America. Bolettino della Societa Paleontologica Italica, 34:205-221. Azzaroli, A., and M.R. Voorhies. 1990. The genus Equus in North America: the Blancan species. Palaeontographica Italica, 80:175-198. Azzaroli, A., C. De Giuli, G. Ficcarelli, and D. Torre. 1988. Late Pliocene to early midPleistocene mammals in Eurasia: faunal succession and dispersal events. Palaeogeography, Palaeoclimatology, Palaeoecology, 66:77-100. Bader, R.S. 1956. A quantitative study of the Equidae of the Thomas Farm Miocene. Bull. Mus. Comp. Zool. 115:47-78. Badgley, C. and L. Tauxe. 1990. Paleomagnetic stratigraphy and time in sediments: studies in alluvial Siwalik rocks of Pakistan. Jour. Geol. 98:457-477. Badoux, D.M. 1987. Some biomechanical aspects of the structure of the equine tarsus. Anatomischer Anzeiger, Jena, 164:53-61. Bagtache, B., D. Hadjouis, and V. Eisenmann. 1984. Présence d’un Equus caballin (E. algericus no. sp.) et d’une autre espèce nouvelle d’Equus (E. malkeinsis n. sp.) dans l’Aterian des Allobroges, Algérie. Comptes-Rendus d l’Académie des Sciences, Paris, 298, série II: 609-612. Barbour, E. H. 1914. A new fossil horse, Hypohippus matthewi. Bull. Nebraska Geol. Surv., 4(10):169-173, pl. I (H. matthewi). Barry, J.C., E.H. Lindsay, and L.L. Jacobs. 1982. A biostratigraphic zonation of the middle and upper Siwaliks of the Potwar Plateau of northern Pakistan. Palaeogeography, Palaeoclimatology, Palaeoecology, 37:95-130. Benirschke, K., N. Malouf, R.J. Low, and H. Heck. 1965. Chromosome complement: difference between Equus caballus and Equus przewalskii Poliakoff. Science, 148:382383. Bennett, D.K. 1980. Stripes do not a zebra make, Part I: A cladistic analysis of Equus. Syst. Zool., 239(2): 271-294. Bennett, D.K. 1984. Cenozoic rocks and faunas of north-central Kansas, with an appendix concerning taxonomy and evolution in the genus Equus. The University of Kansas, Dept. Syst. and Ecol., dissertation submitted for the degree of Doctor of Philosophy. Bennett, D.K. 1988. The ring of muscles. Equus Magazine, 121:36-42. Bennett, D.K. 1992. The evolution of the horse, in J.W. Evans, ed., Horse Breeding and Management, World Encyclopedia of Animal Science, Vol. C7. Elsevier Publ., Amsterdam, pp. 1-40. Benton, M.J. 1990. Vertebrate Palaeontology. Unwin Hyman Publ., London. Berggren, W.A. and J.A. Van Couvering. 1974. The late Neogene: biostratigraphy, geochronolgy and paleoclimatology of the last 15 mmillion years in marine and continental sequences. Palaeogeography, Palaeoclimatology, Palaeoecology. 16:1-216. Bernour, R.L. and Hussain, S.T. 1985. An assessment of the systematic, phylogenetic and biogeographic relationships of Siwalik hipparionine horses. Jour. Vert. Paleo. 5:32-87. Bernour, R.L. and H. Tobien. 1989. Two small species of Cremohipparion (Equidae, Mamm.) from Samos, Greece. Mitt. Bayer. Staatsslg. Palaont. Hist. Geol. 29:207-226. Berry, W.B.N. 1987. Growth of a Prehistoric Time Scale: Based on Organic Evoluton. Blackwell Scientific Publl, Palo Alto, California. Bock, W.J. 1973. Philosophical foundations of classical evolutionary classification. Syst. Zool., 22:375-392. Boddaert, P. 1785. Elenchus Animalium. C.R. Hake, Roterodami [Rotterdam], Sistens huc usque nota, eorumque varietates, I: 1-173. Boessnick, J. 1970. Ein altaegyptisches Pferdeskelett. Mitteilungen Deutschen Archaeologischen Institut (Kairo), 26:43-47. Boessnick, J. and A. Von den Driesch. 1976. Pferde im 4./3. Jahrtausend v. Chr. In Ostanatolien. Säugetierkundliche Mitteilungen, 24:82-87. Bonaparte, J.F. 1986. A new and unusual late Cretaceous mammal from Patagonia. Jour. Vert. Paleontol. 6:264-270. Boné, E.L. and R. Singer. 1965. Hipparion from Langebaanweg, Cape Province and a revision of the genus in Africa. Ann. South African Mus. 48:273-397. Boulé, M. 1899. Observations sur queleques equides fossiles. Bull. Soc. Geol. France 3:27. Bowler, P.J. 1986. Fossils and Progress: Paleontology and the Idea of Progressive Evolution in the Nineteenth Century. Science History Pubglications, New York. Bowler, P.J. 1989. Holding your head up high: degeneration and orthogenesis in theories of human evolution, in J.R. Moore, ed., History, Humanity, and Evolution. Cambridge University Press, pp. 329-353. Branco, W. 1883. Eine fossile Saugethier-Fauna von Punin bei Riobamba in Ecuador. Pal. Abh. 1:2. Brentjes, B. 1972. Das Pferd im Alten Orient. Säugetierkundliche Mitteilungen, 20:325353. Brincken, J. (Baron de). 1828. Mémoire Descriptif sur la Forêt Impériele de Bialowieza, en Lithuanie. N. Glücksberg, Imprmeur – Libraire de l’Université Royale, Varsovie [Warsaw], 127 pp. Brooks, C.E.P. 1928. Climate Through the Ages: A Study of the Climatic Factors and their Variation. Yale University Press, New Haven. Broom, R. 1909. On the evidence of a large horse recently extinct in South Africa. Ann. South Afr. Mus., Kapstadt, 7:281-282. Brown, T.M. and A.J. Kihm. 1981. Xenicohippus, an unusual new hyracothere (Mammalia, Perissodactyla) from lower Eocene rocks of Wyoming, Colorado, and New Mexico. Jour. Paleontol. 55:257-270. Brown, W.L. Jr. 1987. Punctuated equilibria excused: the original examples fail to support it. Biol. Jour. Linnean Soc. 31:383-404. Burmeister, H. 1875. Los Caballos Fòsiles de la Pampa Argentina. La Tribuna Press, Buenos Aires, Argentina. Butler, P.M. 1952a. The milk-molars of Perissodactyla, with remarks on molar occlusion. Proc. Zool. Soc. London 121:777-817. Butler, P.M. 1952b. Molarisation of premolars in Perissodactyla. Proc. Zool. Soc. London, 121:819-843. Cabrera, A. 1936. Subspecific and individual variation in the Burchell zebras. Jour. Mamm. 17:89-112. Cain, A.J. 1954. Animal Species and Their Evolution. Hutchinson’s University Library, London. Camp, C.L. and N. Smith. 1942. Phylogeny and functions of the digital ligaments of the horse. Univ. California Mem. 13:69-124. Carroll, R.L. 1988. Vertebrate Paleontology and Evolution. Freeman Co., New York. Carter, D.C. 1984. Perissodactyls, in S. Anderson and J. K. Jones, Jr., eds., Orders and Families of Recent Mammals of the World. John Wiley and Sons, New York, pp. 549562. Cerling, T.E., J. Quade, Y. Wang, and J.R. Bowman. 1989. Carbon isotopes in soils and paleosols as ecology and paleoecology indicators. Nature 341:138-9. Chaney, R.W. and Elias, M.K. 1926. Late Tertiary floras from the High Plains. Carneg. Inst. Wash. Publ. 476:1-72. Chow, M. and T. Qi. 1978. Paleocene mammalian faunas from Nomogen formation of Inner Mongolia. Vert. Palasiatica 16:77-85. Christol, A. de 1832. [No title; description of Hipparion]. Ann. Sci. Indust. du Midi de France, Vol. I:180-181. Chubb, S.H. 1934. Frontal protuberances in horses: an explanation of the so-called “horned” horse. Am. Mus. Novitates 740:1-9. Churcher, C.S. and A. McS. Stalker. 1970. A late, postglacial horse from Pashley, Alberta. Canadian Jour. Earth Sci., 7:1020-1026. Churcher, C.S. 1982. Oldest ass recovered from Olduvai Gorge, Tanzania, and the origin of asses. Jour. Paleo. 56(5):1124-1132. Churcher, C.S., and M.L. Richardson. 1978. Equidae, in: V.J. Maglio and H.B.S. Cooke, eds., Evolution of African Mammals. Harvard University Press, Cambridge, Mass., pp. 379-442. Cifelli, R.L. 1981. Patterns of evolution among the Artiodactyla and Perissodactyla (Mammalia). Evolution 35:433-440. Clark, J., J.R. Beerbower, and K.K. Kietzke. 1967. Oligocene sedimentation, stratigraphy, paleoecology and paleoclimatology in the Big Badlands of South Dakota. Fieldiana: Geol. Mem. 5:1-158. Clutton-Brock, J. 1981. Domesticated Animals from Early Times. University of Texas Press, Austin. Colbert, E.H. 1935. Distributional and phylogenetic studies on Indian fossil mammals. II. The correlation of the Siwaliks of India as inferred by the migrations of Hipparion and Equus. Amer. Mus. Novitates 797:1-15. Colbert, E.H. 1968. Men and Dinosaurs: the Search in Field and Laboratory. Dutton & Co., New York. Colbert, E.H. 1980. Evolution of the Vertebrates: A History of the Backboned Animals Through Time. Wiley & Sons, New York. Cooper, C.F. 1932. The genus Hyracotherium: a revision and description of new specimens found in England. Phil. Trans.Roy. Soc. London, 431:22-48. Cope, E. D. 1873. Third notice of extinct Vertebrata from the Tertiary of the plains, Palaeontological Bull. no. 16: 1-8 (Anchitherium cuneatum, p. 7). Cope, E. D. 1874. Report on the vertebrate paleontology of northern Colorado, Ann. Rept. U.S. Geol. and Geogr. Surv. Terr. embr. Colorado (1873), p. 496 (Anchitherium exoletum); p. 12, no fig. (Hippotherium paniense); p. 13, no fig. (Protohippus labrosus); p. 15, no fig. (Protohippus sejunctus) Cope, E. D. 1875. On some new fossil Ungulata. Proc. Acad. Nat. Sci. Phila. 27:259-260, no fig. (Hippotherium calamarium). Cope, E. D. 1878. On some of the characters of the Miocene fauna of Oregon. Proc. Amer. Philos. Soc. 18(1878-1880), p. 73, no fig. (Anchitherium equiceps); 74-5, no fig (A. brachylophum); 75, no fig. (A. longicristis); p. 76, no fig. (Stylonus seversus). Cope, E. D. 1879. A new Anchitherium. Amer. Nat. 13(7):462-3, no fig. (Anchitherium praestans). Cope, E. D. 1880. A new Hippidium. Amer. Nat. 14(3):223, no fig. (H. spectans). Cope, E. D. 1881. On the origin of the foot structure of the ungulates. Amer. Nat. 15(4):271, fig. 3 (figure of Protohippus sejunctus) Cope, E.D. 1884. The Vertebrata of the Tertiary Formations of the West, Book 1. U.S. Geological Survey, Washington, D.C. Cope, E. D. 1885a. Report on the coal deposits near Lacualtipan, in the State of Hidalgo, Mexico. Proc. Amer. Philos. Soc. 23(1886):150-151, fig. 2 (Protohippus castilli) Cope, E. D. 1885b. Pliocene horses of southwestern Texas, Amer. Nat. 19(12):1208-09, pl. xxxvii, fig. 6 (more on Protohippus castilli) p. 1208-9, no description, pl. 37, fig. 5 (Hippotherium peninsulatum) Cope, E. D. 1886a. On two new species of three-toed horses from the Upper Miocene, with notes on the fauna of the Ticholeptus beds. Proc. Amer. Philos. Soc., 23(1886):359, no fig. (Hippotherium seversum); p. 360-361, no fig. (Hippotherium rectidens); p. 357-358, no fig. (Anchitherium ultimum) Cope, E. D. 1886b. Report on the coal deposits near Lacualtipan, in the State of Hidalgo, Proc. Amer. Philos. Soc. 23:150, fig. 1 (description of Hipparion peninsulatum) Cope, E. D. 1887. The Perissodactyla. Amer. Nat. 21(12):1069, fig. 36. Cope, E. D. 1889a. An intermediate Pliocene fauna. Amer. Nat. 23(268):253-4, no fig. (Hippotherium relictum). Cope, E. D. 1889b. A review of the North American species of Hippotherium. Proc. Amer. Philos. Soc. 26(1889):449, figs. 19, 19a, 20 (more on H. relictum); more on Stylonus seversus; p. 449, no fig. (Hippotherium sphenodus); p. 446, figs. 7, 7a, 8 (Hippotherium retrusum); p. 446-7, no fig. (Protohippus or Hippotherium profectus); p. 434-435, figs. 2, 2a (descr. and figs. of Hippotherium sinclairi Wortman) Cope, E. D. 1892. A contribution to the vertebrate paleontology of Texas. Proc. Amer. Philos. Soc. 30(137):124-5, fig. 1 (Equus simplicidens). Cope, E. D. 1893a. On the permanent and temporary dentitions of certain three-toed horses. Amer. Nat. 26(1892):943-4, pl. XXVI, fig. 1 (Protohippus pachyops); see also Cope, 1893b, for description. Cope, E. D. 1893b. A preliminary report on the vertebrate paleontology of the Llano Estacado. Fourth Ann. Rept. Geol. Surv. Texas (1892), pp. 26-29, pl. iii, fig. 1, pl. ix, figs. 2,3 (description of Protohippus pachyops); p. 30-32, pls. v, vi, vii (Protohippus fossulatus); p. 42-43, pl. xii, figs. 3, 3a, 4 (Hippidium interpolatum); p. 67, pl. xx, fig. 7 (Equus cumminsii); p. 67-8, pl. xx, fig. 8 (Equus minutus); p. 41-42, pl. xii, figs. 1,2,2a (Protohippus lenticularis); p. 43-45, pl. xx, figs. 6, 6a (Equus eurystylus) Cope, E.D., and W.D. Matthew. 1915. Tertiary Mammalia and Permian Vertebrata. U.S. Geological Survey and American Museum of Natural History Monograph Series no. 2, plates I-CLIV. Corbet, G.B. and J. Clutton-Brock. 1984. Appendix: taxonomy and nomenclature, in I.L. Mason, ed., The Evolution of Domesticated Animals, pp. 434-438. Cracraft, J. 1981. Patterns and process in paleobiology: the role of cladistic analysis in systematic paleontology. Paleobiology 7:456-468. Crusafont, M., and P. Sondaar. 1971. Une nouvelle espece d'Hipparion du Pliocene terminal d'Espagne. Palaeovertebrata 4: 28-37. Cuvier, F.G. 1825. Des dents des mammiferes, consideres comme caracteres zoologiques, in F.G. Leverault, ed., Dictionnaire des Sciences Naturelles, GUAHEO, Paris, Vol. 20: 1-572. Cuvier, G. 1825a. Recherches sur les ossemens fossiles, ou l'on retablit les caractéres de plusieurs animaux dont les revolutions du globe ont detruit les espéces. Paris. Cuvier, G. 1825b. Des dents des mammiferes, considerés comme carateres zoologiques, in F.G. Leverault (ed.), Dictionnaire des Sciences Naurelles, GUAHEO, Paris, 20:1-572. Cuvier, G. 1836. Recherches sur les Ossemens Fossiles. Edmond D’Ocagne, Paris. Dalquest, W.W. 1965. The Pleistocene horse E. conversidens. Amer. Midl. Nat. 74(2):408-417. Dalquest, W.W. 1967. Mammals of the Pleistocene Slaton local fauna of Texas. Southw. Nat. 12(1):1-30. Dalquest, W.W. 1978. Phylogeny of American horses of Blancan and Pleistocene age. Ann. Zoologica Fennici 15:191-199. Dalquest, W.W. 1988. Astrohippus [sic] and the origin of Blancan and Pleistocene horses. Occas. Pap. Mus. Texas Tech. Univ. 116:1-23. Dalrymple, G.B. 1979. Critical tables for conversion of K-Ar ages from old to new constants. Geology 7:558-560. Dalrymple, G.B. and M.A. Lanphere. 1969. Potassium-Argon Dating: Principles, Techniques, and Applications to Geochronology. Freeman & Co., San Francisco. Darlington, P.J. 1957. Zoogeography: the Geographical Distribution of Animals. Wiley & Sons, New York. Darwin, Charles. 1852. Journal of Researches into the Natural History and Geology of the Countries Visited During the Voyage of the H.M.S. Beagle Round the World, new ed. Murray Co., London. Darwin, Charles. 1859. On the Origin of Species (a facsimile of the first edition, with introduction by Ernst Mayr, 1964). Harvard University Press, Cambridge, Massachussetts. Dashzeveg, D. 1979. On an archaic representative of the equoids (Mammalia, Perissodactyla) from the Eocene of central Asia. Trans. Joint Soviet-Mongolian Paleontol. Exped. 8:10-22. Davis, S.J. 1980. Late Pleistocene and Holocene equid remains from Israel. Zool. Jour. Linn. Soc., 70-289-312. De Cristol, A. 1832. Fossil horse remains from Mt. Leberon, southern France. Ann. Sci. Indust. Du Midi de France, Vol. 1: 215. Déperet, C. 1917. Monographie de la faune de mammiferes fossiles du Ludien inferieur d’Euzetles-Bain (Gard). Ann. L’Univ. Lyon, I, Sciences, Medecine, 40:1-274. De Porta, J. 1960. Los equidos fósiles de la Sabana de Bogotá. Bol. Geol., Univ. Industrial Santander (Colombia) 4:51-78. Desmond, A. 1982. Archetypes and Ancestors: Palaeontology in Victorian London 18501875. The University of Chicago Press, Chicago. Devillers, C. J. Mahe, D. Ambrose, R. Bauchot, and E. chatelain. 1984. Allometric studies on the skull of living and fossil Equidae (Mammalia, Perissodactyla). Jour. Vert. Paleontol. 4:471-480. Dimery, N.J., R. McN. Alexander, and K.A. Deyst. 1985. Mechanics of the ligamentum nuchae of some artiodactyls. Jour. Zool. (London), 206:341-351. Dimery, N.J., R. McN. Alexander, and R.F. Kerr. 1986. Elastic extension of leg tendons in the locomotion of horses (Equus caballus). Jour. Zool. London, 210:415-425. Donoghue, M.J., J.A. Doyle, J. Gauthier, A.G. Kiluge, and T. rowe. 1989. The importance of fossils in phylogeny reconstruction. Ann. Rev. Ecol. Syst. 20:431-460. Douglass, E. 1899. The Neocene lake beds of western Montana and descriptions of some new vertebrates from the Loup Fork, Th. for the Degree of M.S., Univ. Montana, June, 1899, pp. 26-27, no fig. (A[n]chitherium minimus). Douglass, E. 1908. Fossil horses from North Dakota and Montana. Ann. Carneg. Mus., 4 (3 and 4):268-9, pl. lxv, figs. 2 and 3. (Mesohippus portentus); pp. 271-273, pl.. LXVII, figs. 3-4, pl. lxvii, figs. 6-8 (Altippus taxus); p. 274, pls. lxvi, lxviii, figs. 1-2 (Merychippus ? missouriensis) Downs, T. 1961. A study of variation and evolution in Miocene Merychippus. Los Angeles Co. Mus. Contrib. Sci. 45:1-75. Duerst, J.U. 1908. Animal remains from the excavations at Anau and the horse of Anau in its relation to the races of domestic horse. Explorations in Turkmenistan, vol. II. Durham, J.W. 1959. Paleoclimates, in L.H. Ahrens (ed.), Physics and Chemistry of the Earth, Vol. 3. Pergamon Press, New York. Edinger, Tilly. 1948. Evolution of the horse brain. Mem. Geol. Soc. Amer., 25:1-92. Edinger, Tilly. 1950. Frontal sinus evolution (particularly in the Equidae). Bull. Mus. Comp. Zool., Harvard University, 103:412-496. Edinger, Tilly and D.B. Kitts. 1954. The foramen ovale. Evolution, 8:389-404. Eisenberg, J.F. 1981. The Mammalian Radiations: An Analysis of Trends in Evolution, Adaptations, and Behavior. The University of Chicago Press, Chicago. Eisenmann, V. 1975. Nouvelles interprétations des restes d’Equidés (Mammalia, Perissodactyla) de Nihowan (Plèistocéne Inférieur de la Chine du Nord): Equus teilhardi nov. sp. Géobios 8:125-134. Eisenmann, V. 1977. Les Hipparions africains: Valeur et signification de quelques caractères des jugales infèrieures. Bull. Mus. Natl. Hist. Nat. (France) 438:69-86. Eisenmann, V. 1986. Comparative osteology of modern and fossil horses, half-asses, and asses, in R.H. Meadow and H.-P. Uerpmann, eds., Equids in the Ancient World. L. Reichart Verlag, Wiesbaden, pp. 67-116. Eisenmann, V., and J.C. Turlot. 1978. Sur la taxonomie du genre Equus: description et discrimination des espéces actuelles d'après les données craniométriques. Cahiers de l'a’alyse des Donées V., 3:179-201. Eisenmann, V. 1980. Les chevaux (Equus sensu lato) fossiles et actuels: crânes et dents jugales supèrieures. Cahiers de Paleont., Cent. Natl. Recherche Sci. 1-186. Eisenmann, V. 1981. Etude des dents jugales infèrieures des Equus (Mammalia, Perissodactyla) actuels et fossiles. Palaeovertebtrata 10:127-226. Eisenmann, V. 1986. Comparative osteology of modern and fossil horses, half-asses, and asses, in r.H. Meadow and H.-P. Uerpman, eds., Equids in the Ancient World. Dr. Ludwig Reichart Verlag, pp. 67-116. Eisenmann, V., E. Cregut-Gonnoure, and A.-M. Moigne. 1985. Equus mosbachensis et les grands chevaux de la Caune l’Arago et de Lunel-Viel: craniologie comparée. Bulletin du Muséum National d’Histoire Naturelle, Paris, 4e, ser. 7, sect. C, no. 2:157-173. Elderfield, H. 1986. Strontium isotope stratigraphy. Palaeogeography, Palaeoclimatology, Palaeoecology. 57:71-90. Eldridge, Niel and Stephen Jay Gould. 1972. Punctuated equilibria: an alternative to phyletic gradualism, in T.J.M. Schopf (ed.), Models in Paleobiology. Freeman, Cooper and Co., San Francisco. Emry, R.J., L.S. Russell, and P.J. Bjork. 1987. The Chadronian, Orellan, and Whitneyan North American Land Mammal Ages, in M.O. Woodburne, ed., Cenozoic Mammals of North America: Geochronology and Biostratigraphy, pp. 118-152. Evander, R.LO. 1989. Phylogeny of the family Equidae, in D.R. Prothero and R.M. Schoch, eds., The Evolution of Perissodactyls. Clarendon Press, Oxford, pp. 109-127. Evernden, J.F., D.E. Savage, G.H. Curtis, and J.T. James. 1964. Potassium-argon dates and the Cenozoic mammalian chronology of North America. Amer. Jour. Sci. 262:145198. Ewart, J.C. 1894. The development of the skeleton of the limbs of the horse, with observations on polydactyla. J. Anat. Physiol. London, N.S., 28: 18-44. Falconer, H., and P. Cautley. 1845-1849. Fauna Antiqua Sivalensis, Being the Fossil Zoology of the Siwalik Highlands in the North of India. Smith, Elder, and Co., London, Part 9: 1-92. Filhol, H. 1888. Etudes sur les vertebres fossiles d'Issel (Aude). Mem Soc. Geol. France (3)5:1-188. Firouz, L. 1973. Osteological and historical implications of the Caspian miniature horse to early horse domestication in Iran, in J. Matolsci, ed., Domestikationsforschung und Geschichte der Haustiere. Akademii Kiado, Budapest, pp. 309-315. Fischer, M.S. 1989. Hyracoids, the sister-group of Perissodactyls, in D.R. Prothero and R.M. Schoch, eds., The Evolution of Perissodactyls. Clarendon Press, Oxford, pp. 37-56. Flerov, C. 1931. Quelques données sur la craniologie de la famille des Equidae. C.R. Acad. Sci. U.R.S.S. 10:269-272. Flower, W.H. 1892. The Horse: A Study in Natural History. D. Appleton & Co., New York. Flynn, J.J. 1986. Faunal provinces and the Simpson coefficient, in K.M. Flanagan and J.A. Lillegraven, eds., Vertebrates, Phylogeny, and Philosophy. Univ. Wyo. Contrib. Geol., spec. Pap. 3, pp. 317-338. Forstén, A.-M. 1968. Revision of the Palearctic Hipparion. Acta Zool. Fennica, 119: 1-134. Forstén, A.-M. 1970. Variation in and between three populations of Mesohippus bairdii Leidy from the Big Badlands, South Dakota. Acta Zool. Fennica 126:1-16. Forstén, A.-M. 1973. Size and shape evolution in the cheek teeth of fossil horses. Acta Zool. Fennica. 137:1-31. Forstén, A.-M. 1975. The fossil horses of the Texas Gulf Coastal Plain: a revision. Pearce-Sellards Ser., Texas Mem. Mus. 22:1-86. Forstén, A.-M. 1982a. The taxonomic status of the Miocene horse genus Sinohippus. Palaeontology 25:673-679. Forstén, A.-M. 1982b. The status of the genus Cormohipparion Skinner and MacFadden (Mammalia, Equidae). Jour. Paleontol. 56:1332-1335. Forstén, A.-M. 1983. The preorbital fossa as a taxonomic character in some Old World Hipparion. Jour. Paleo., 57:686-704. Forstén, A.-M. 1986. Chinese fossil horses of the genus Equus. Acta Zool. Fennica 181:1-40. Forstén, A.-M. 1988a. Middle Pleistocene replacement of stenonid horses by caballoid horses – ecological implications. Palaeogeography, Palaeoclimatology, Palaeoecology, 65:23-33. Forstén, A.-M. 1988b. The small caballoid horse of the upper Pleistocene and Holocene. Zeitschrift fur Tierzuchtung und Zuchtungsbiologie, 105:161-176. Forstén, A.-M. 1989. Horse diversity through the ages. Biological Review, 64:279-304. Forstén, A.-M. and V. Dimitrijevic. 1995. The Palaeolithic horses (genus Equus) from Risovaca, Arandjelovac, Serbia. Neues Jahrbuch der Geologischen und Paläontologischen Abhandlungen, 196:395-409. Forster-Cooper, C. 1932. The genus Hyracotherium: a revision and description of new specimens found in England. Phil. Trans. Roy. Soc. London, ser B 221:431-448. Forsyth-Major, C.J. 1880. Beitrage zur Geschichte der fossilen Pferde. Abh. Schweiz. pal. Ges. 7. Franzen, J.L. 1985. Exceptional preservation of Eocene vertebrates in the lake deposit of Grube Messel (West Germany). Phil. Trans. Roy. Soc. London, Ser. B 311:181-186. Fortelius, M. 1985. Ungulate cheek teeth: developmental, functional, and evolutionary interrelations. Acta Zool. Fennica, 180:1-76. Frick, Childs. 1937. Horned Ruminants of North America. Bull. Amer. Mus. Nat. Hist. 69:1-669. Froelich, J. 1989. A small hyracothere from the San Jose Formation (Lower Eocene) of New Mexico. Jour. Vert. Paleontol. 9:21A. Gabounia, L.K. 1959. Histoire des Hipparions. Nauk. Acad. S.S.S.R. Gaffney, E.S. 1979. An introduction to the logic of phylogeny reconstruction, in J. Cracraft and N. Eldredge, eds., Phylogenetic Analysis and Paleontology. Columbia University Press, New York, pp. 79-111. Gao Xingyi, and Gu Jinghe. 1989. [The distribution and status of the Equidae in China]. Acta Therological Sinica, 9:269-274 (in Chinese, English summary). Garrutt, E.W., I.I. Sokolow, and T.N. Salesskaja. 1966. Erforshcung und Zucht des Przewalski-Pferdes (Equus przewalskii Poljakoff) in der Sowjetunion. Zeitschrift für Tierzüchtung und Züchtungsbiologie, 82:377-426. Gaudry, A. 1867. Animaux Fossiles et Geologie de l’Attique, 4th ed. Paris. Gaudry, A. 1896. Essai de Paleontologie Philosophique. Masson, Paris (1980 facsimile reprint by Arno Press, New York). Gazin, C.L. 1936. A study of the fossil horse remains from the Upper Pliocene of Idaho. Proc. U.S. Natl. Mus. 83:281-319 (Equus shoshonensis) George, M., Jr. and O.A. Ryder. 1986. Mitochondrial DNA evolution in the genus Equus. Molecular Biology and Evolution, 3:535-546. George, T.N. 1956. Biospecies, chronospecies, and morphospecies. Syst. Assoc. Publ. 2:123-137. Gidley, J.W. 1903. A new three-toed horse. Bull. Amer. Mus. Nat. Hist. 19(8):467-476, no fig. (Neohipparion whitneyi) Gidley, J.W. 1906. A new genus of horse from the Mascall beds, with notes on a small collection of equine teeth in the University of California, Bull. Amer. Mus. Nat. Hist. 22(22):385-388, figs. A,B (Archaeohippus, gen. nov.) Gidley, J.W. 1907. Revision of the Miocene and Pliocene Equids of North America. Bull. Amer. Mus. Nat. Hist. 23(25):932, no fig. (Parahippus pawniensis); p. 932, no fig. P. coloradensis); p. 928, no fig. (Merychippus campestris); p. 930-1, no fig. (Hypohippus osborni) Gillette, D.D. and E.H. Colbert. 1976. Catalog of type specimens of fossil vertebrates, Academy of Natural Sciences, Philadelphia, Part II: Terrestrial mammals. Proc. Acad. Nat. Sci. 128:25-28. Gingerich, P.D. 1976. Paleontology and phylogeny: patterns of evolution at the species level in early tertiary mammals. Amer. Jour. Sci. 276:1-28. Gingerich, P.D. 1977. Patterns of evolution in the mammalian fossil record, in A. Hallam, ed., Patterns of Evolution as Illustrated by the Fossil Record. Elsevier Publishing, Amsterdam, pp. 469-500. Gingerich, P.D. 1981. Variation, sexual dimorphism, and social structure in the early Eocene horse Hyracotherium (Mammalia, Perissodactyla). Paleobiology 7:443-455. Gingerich, P.D. 1983. Rates of evolution: effects of time and temporal scaling. Science, 222:159-161. Goldschmidt, R. 1940. The Material Basis of Evolution. Yale University Press, New Haven. Gould, S.J. 1983. Hen’s Teeth and Horse’s Toes. Norton Press, New York. Gould, S. J. 1989. Wonderful Life: the Burgess Shale and the Nature of History. Norton Press, New York. Gould, S.J. 1991. Fall in the House of Ussher: How foolish was the archbishop’s precise date for Creation? Nat. Hist. 100(11):12-21. Gould, S.J. and N. Eldredge. 1977. Punctuated equilibria: the tempo and mode of evolution reconsidered. Paleobiology 3:115-151. Gould, S.J. and R.C. Lewontin. 1979. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. Royal Soc. Lond., Ser. B, 205(581-598). Graham, R.W., and E.L. Lundelius, principal authors. 1994. FAUNMAP: a database documenting late Quaternary distributions of mammal species in the United States. Illinois State Museum Scientific Papers, 1:1-287; 2:289-690. Granger, W.A. 1908. A revision of the American Eocene horses. Bull. Amer. Mus. Nat. Hist. 24:221-164. Gray, J.E. 1821. On the natural arrangement of vertebrose animals. London Med. Reposit. Rev. 15:296-310. Grayson, D.K. 1989. The chronology of North American late Pleistocene extinctions. Jour. Archaeol. Sci. 16:153-166. Gregory, J.T. Pliocene vertebrates from Big Spring Canyon, South Dakota. Univ. Calif. Publ., Dept. Geol. Sci. 26:307-446. Gregory, W.K. 1910. The orders of mammals. Bull. Amer. Mus. Nat. Hist. 27:1-524. Gregory, W.K. 1920. Studies in comparative myology and osteology, no. V: On the anatomy of the preorbital fossae of Equidae and other ungulates. Bull. Amer. Mus. Nat. Hist. 42:265-284. Gromov, I.M. and G.I. Baranova (eds.). 1981. Katalog mlekopitayushchikh SSSR, Pliosten-Sovremenost’ [Catalog of mammals of the USSR, Pliocene-Recent]. Nauka, Leningrad, pp. 1-456. Gromova, V. 1949. Histoire des chevaux (genre Equus) de l'Ancien Monde. Trav. Inst. Paleontol. Acad. Sci. U.R.S.S. 7:1-373. (French transl. by P. de St. Auboin, Ann. Cent. Etud. Docum. Paleont. 13, Paris, 1955). Gromova, V. 1952. Le genre Hipparion. Bur. Res. Min. Geol. 12:1-288. Gromova, V. 1959. O skelete tarpana (Equus caballus gmelini Ant.) I drugikh sovremennykh dikikh loshadei (chast’ I)[On the skeleton of the tarpan … and other contemporary wild horses (part I)]. Bulletin Moskovskovo obshchestva ispytatelei prirody, otdel Biologii, novaya seriya, 64:99-124. Groves, C.P., and V. Mazak. 1967. On some taxonomic problems of Asiatic wild asses, with the description of a new subspecies (Perissodactyla: Equidae). Sonderdrück aus Z.F. Saugetierkund. 32(6):321-355. Groves, C.P. 1974. Horses, Asses, and Zebras in the Wild. Ralph Curtis books, Hollywood, Florida, pp. 1-192. Groves, C.P. 1986. The taxonomy, distribution, and adaptations of recent equids, in R.H. Meadow and H.-P. Uerpmann, eds., Equids in the Ancient World. L. Reichert Verlag, Wiesbaden, pp. 11-51. Groves, C.P. and D.P. Willoughby. 1981. Studies on the taxonomy and phylogeny of the genus Equus: subgeneric classification of the Recent species. Mammalia, 45:321-354. Guthrie, R.D. 1990. Frozen Fauna of the Mammoth Steppe. University of Chicago Press, pp. 1-323. Higuchi, R., B. Bowman, M. Freiberger, O. Ryder, and A.C. Wilson. 1984. DNA sequences from the quagga, an extinct member of the horse family. Nature 312:282-284. Harris, J.M. and G.T. Jefferson, eds. 1985. Rancho La Brea: Treasures of the Tar Pits. Los Angeles Co. Nat. Hist. Mus. Sci. Ser. No. 31. Hay, O.P. 1902. Bibliography and catalogue of the fossil Vertebrata of North America. Bull. U.S. Geol. Surv. 179:1-868. Hay, O.P. 1913a. Description of the skull of an extinct horse, found in Central Alaska. Smithsonian Misc. Coll., 61:1-18. Hay, O.P. 1913b. Notes on some fossil horses, with descriptions of four new species. Proc. United States Nat. Mus., 44:5769-594. Hennig, W. 1966. Phylogenetic Systematics. Univ. Illinois Press, Urbana. Heptner, V.G. 1961. Distribution, geographical variability and biology of wild horses on the territory of the U.S.S.R. Equus (Prague), 1:28-41. Hermanson, J.W., and B.J. MacFadden. 1992. Evolutionary and functional morphology of the shoulder region and stay apparatus in fossil and extant horses (Equidae). Jour. Vert. Paleo. 12:377-386. Hibbard, Claude W. 1955. Pleistocene vertebrates from the upper Becarra (Becarra Superior) formation, valley of Tequixquiac, Mexico, with notes on other Pleistocene forms. Contrib. Mus. Paleontology, Univ. Michigan, 12:47-96. Hildebrand, M. 1965. Symmetrical gaits of horses: gaits can be expressed numerically and analyzed graphically to reveal their nature and relationship. Science, 150:701-708. Hildebrand, M. 1974. Analysis of Vertebrate Structures. John Wiley & Sons, New York. Hilzheimer, M. 1935. The evolution of the domestic horse. Antiquity 9. Hoffstetter, R. 1950. Some observations on the fossil horses of South America, Amerhippus gen. nov. Bolivian Information Service, Natural History, Ecuador. 3:426-454. Hoffstetter, R. 1952. Les Mammifères Pléistocènes de la Rèpublique de L’Equaterur. Soc. Géol. France. Mém. 66:1-391. Honacki, J.H., K.E. Kinman, and J.W. Koeppl, eds. 1982. Mammal Species of the World: A Taxonomic and Geographic Reference. Allen Press and the Association of Systematic Collections, Lawrence, Kansas, pp. 308-311. Hooker, J.J. 1984. A primitive ceratomorph (Perissodactyl, Mammalia) in the English early Eocene. Bull. Brit. Mus. (Nat. Hist.), Geol. 33:101-14. Hooker, J.J. 1989. Character polarities in early Perissodactyls and their significance for Hyracotherium and infraordinal relationships, in D.R. Prothero and R.M. Schoch, eds., The Evolution of Perissodactyls. Clarendon Press, Oxford, pp. 79-101. Hopwood, A. 1936. The former distribution of caballine and zebrine horses in Europe and Asia. Proc. Zool. Soc. London, 897-912. Hoyt, d.F., and C.R. Taylor. 1981. Gait and the energetics of locomotion in horses. Nature, 292:239-240. Hsü, K.J. 1986. The Great Dying: Cosmic Catastrophe, Dinosaurs, and the Theory of Evolution. Ballantine Books, New York. Hulbert, R.C. Jr., 1982. Population dynamics of the three-toed horse Neohipparion from the late Miocene of Florida. Paleobiology 8:159-167. Hulbert, R.C., Jr. 1984. Paleoecology and population dynamics of the early Miocene (Hemingfordian) horse Parahippus leonensis from the Thomas Farm site, Florida. Jour. Vert. Paleontol. 4:547-558. Hulbert, R.C. Jr. 1987. A new Cormohipparion (Mammalia, Equidae) from the Pliocene (latest Hemphillian and Blancan) of Florida. Jour. Vert. Paleontol. 7:451-468. Hulbert, R.C. Jr. 1988a. Calippus and Protohippus (Mammalia, Perissodactyla, Equidae) from the Miocene (Barstovian-early Hemphillian) of the Gulf Coastal Plain. Bull. Florida State Mus., Biol. Sci. 32:221-340. Hulbert, R.C. Jr. 1988b. Cormohipparion and Hipparion (Mammalia, Perissodactyla, Equidae) from the late Neogene of Florida. Bull. Florida State Mus., Biol. Sci. 33:229338. Hulbert, R.C. Jr. 1989. Phylogenetic interrelationships and evolution of North american late Neogene Equidae, in D.R. Prothero and R.M. Schoch, eds., The Evolution of Perissodactyls. Clarendon Press, Oxford, pp. 176-196. Hulbert, R.C. Jr. and B.J. MacFadden. 1991. Morphological transformation and cladogenesis at the base of the adaptive radiation of Miocene hypsodont horses. Amer. Mus. Novitates 3000:1-61. Hunt, R.M., Jr. 1990. Taphonomy and sedimentology of Arikareean (lower Miocene) fluvial, eolian, and lacustrine paleoenvironments, Nebraska and Wyoming: a paleobiota entombed in fine-grained volcaniclastic rocks, in M. G. Lockley and A. Rice, eds., Volcanism and Fossil Biotas. Geol. Soc. Amer. Spec. Pap. No. 244, pp. 69-111. Hussain, S.T. 1971. Revision of Hipparion (Equidae, Mammalia) from the Siwalik Hills of Pakistan and INdia. Bayerische Acad. der Wiss., Math., naturw. Klasse, 147:1-68. Hussain, S. T. 1975. Evolutionary and functional anatomy of the pelvic limb in fossil and recent Equidae (Perissodactyla, Mammalia). Anat., Histol., Embryol. 4:179-222. Hutchinson, G.E. 1959. Homage to Santa Rosalia, or why are there so many kinds of animals? Amer. Nat. 93:145-159. Janis, C.M. 1976. The evolutionary strategy of the Equidae and the origins of rumen and cecal digestion. Evolution 30:757-774. Janis, C.M. 1984. The use of fossil ungulate communities as indicators of climate and environment, in P. Brenchley, ed., Fossils and Climate. Wiley & Sons, New York, pp. 85-104. Janis, C.M. 1988. An estimation of tooth volume and hypsodonty indices in ungulate mammals, and the correlation of these factors with dietary preference, in D.E. Russell, J.P. Santiago, and D. Sigogneau-Russell, eds., Teeth Revisited: Proceedings VIIth International Symposium Dental Morphology, Paris 1986. Mem Mus. Natn. Hist. Nat., Paris, Ser C., pp. 367-387. Janis, C.M. and M. Fortelius. 1988. On the means whereby mammals achieve increased functional durability of their dentitions, with special reference to limiting factors. Biol. Rev. 63:197-230. Jepsen, G.L., E. Mayr, and G.G. Simpson, eds. 1949. Genetics, Paleontology, and Evolution. Princeton: Princeton University Press. Johnson, N.M., N.D. Opdyke, and E.H. Lindsay. 1975. Magnetic polarity stratigraphy of Plio-Pleistocene terrestrial deposits and vertebrate faunas, San Pedro Valley, Arizona. Geol. Soc. Amer. Bull. 86:5-12. Kavanaugh, D.H. 1972. Hennig’s princiles and methods of phylogenetic systematics. Biologist, 54:115-127. Kitts, D.B. 1956. American Hyracotherium (Perissodactyla, Equidae). Bull. Amer. Mus. Nat. Hist. 110:1-60. Kitts, D.B. 1957. A revision of the genus Orohippus (Perissodactyla, Equidae). Amer. Mus. Novitates, 1864:1-40. Koenigswald, G.H.R. von. 1970. Hipparion from the Pleistocene of Europe, especially from the Red Crag of East Anglia. Palaeogeography, Palaeoclimatology, Palaeoecology 8(2-3). Kovacs, I. 1971. A systematic description of dental roots, in A.A. Dahlberg, ed., Dental Morphology and Evolution. Univ. Chicago Press, pp. 211-256. Kovalewsky, W. 1873. Sur l'Anchitherium aurelianense Cuv., et sur l'histoire paleontologique des chevaux. Mem. Imp. Acad. Sci. St. Petersburg, 20(5):1-73. Kurtén, Bjorn. 1953. On the variation and population dynamics of fossil and Recent mammal popul.ations. Acta Zool. Fennica 76:1-122. Kurtén, Bjorn. 1968. Pleistocene Mammals of Europe. Aldine Publishing Co., Chicago, pp. 1-316. Kurtén, Bjorn and Elaine Anderson. 1980. Pleistocene Mammals of North America. Columbia Univ. Press, New York, pp. 1-442. Kuz'mina, I.E. 1977. O prosiskhozhedenii i istorii teriofauny Sibirskoi arktiki, in: A.A. Strelkov, ed., Fauna i flora Antropogena severo-vostoka Sibiri, "Nauka", Leningrad, pp. 18-55. Kummel, B. 1970. History of the Earth. W.H. Freeman & Co., San Francisco. Lambe, L.E. 1905. Fossil horses of the Oligocene of the Cypress Hills, Assiniboia. Trans. Roy. Soc. Canada, 2nd ser., 11(4):46, pl. ii, fig. 2 (Mesohippus praecocidens); 47, pl. ii, figs. 3,4 (M. propinquus); 48, pl. ii, figs. 6, 6a, 6b (M. stenolophus); 49, pl. ii, fig. 7 (M. planidens); 50, pl. ii, figs. 8, 8a, 8b (M. assiniboiensis). Lance, J.F. 1950. Paleontologìa y estratigráfia del Plioceno de Yepómera, estado de Chihuahua: 1st parte: Equidos, excepto Neohipparion. Univ. Nac. Autónoma México, Inst. Geol. Bol. Núm. 54:1-81. Lavocat, R. 1958. Classification des Ongules d'apres leur origine et leur evolution. Mammalia 22(1). Lazarev, P. 1980. Antoropogenovye loshadi Yakutii [Anthropogene horses of Yakutiya]. Nauka, Moscow, pp. 1-190. Leidy, J.T. 1847. On the fossil horse of North America. Proc. Acad. Nat. Sci. 3:262-266. Leidy, J.T. 1851a. Palaeotherium bairdii, Proc. Acad. Nat. Sci. Phila., 5(6):122. Leidy, J.T. 1851b. [no title; description of Palaeotherium proutii]. Proc. Acad. Nat. Sci. 5:122, 170-171. Leidy, J.T. 1854. Remarks on Sus americanus, or Harlanus americanus, and on other extinct mammals. Proc. Acad. Nat. Sci. Phila. 7(3), p. 90, no fig. (Hippodon speciosus) Leidy, J.T. 1856. Notices of some remains of extinct Mammalia, recently discovered by Dr. F.V. Hayden, in the bad lands of Nebraska, Proc. Acad. Nat. Sci. Phila. 8(1):59, no fig. (Hipparion occidentale). Leidy, J.T. 1857. Notices of extinct Vertebrata discovered by F.V. Hayden, during the expedition to the Sioux Country under the command of Lieut. G.K. Warren. Proc. Acad. Nat. Sci. Phila. 8(1856):311, no fig. (Merychippus insignis). Leidy, J.T. 1858. Notice of remains of extinct Vertebrata, from the valley of the Niobrara River, collected during the Exploring Expedition of 1857, in Nebraska, under the command of Lieut. G.K. Warren, U.S. Top. Eng., by Dr. F.V. Hayden, Geologist to the Expedition. Proc. Acad. Nat. Sci. Phila., 10(1858):26, no fig. (Anchitherium (Parahippus) cognatus) p. 26-27 (Protohippus gen. nov.); p. 26-27, no fig. (Protohippus perditus); p. 27, no fig. (Pliohippus mirabilis); p. 26, no fig. (Anchitherium (Hypohippus) affinis) Leidy, J.T. 1859. Description of vertebrate fossils, in Holmes, F.S., ed., Post-Pliocene fossils of South Carolina, p. 105, pl. xvi, figs. 32-33a (Hippotherium venustum) Leidy, J.T. 1869. The extinct mammalian fauna of Dakota and Nebraska. Jour. Acad. Nat. Sci. Phila., 7 (1869), 296-299, pl. xvii, figs. 3-4 (more on M. insignis); pp. 277-279, pl. xviii, fig. 40 (Protohippus placidus); p. 328, pl. xxvii, figs. 3,4; neotype desig. by Matthew and Gidley, 1906 (Protohippus supremus); p. 286, p. xviii, figs. 20-23 (Hipparion affine); p. 287-88, pl. xviii, fig. 25 (Hipparion gratum); p. 311, pl. xxi, figs. 11, 12 (more on Hypohippus affinis) Leidy, J.T. 1870. Collection of fossils from valley of Bridger Creek, sent by Thomas Condon. Proc. Acad. Nat. Sci. Phila., 22:112, no fig. (Anchitherium condoni). Leidy, J.T. 1873. Contributions to the extinct vertebrate fauna of the western territories. Rept. U.S. Geol. Surv. Terr., F.V. Hayden, U.S. Geologist in charge, 1(1):218, pl. ii, fig. 5 (first figures of Anchitherium condoni); p. 250-1, pl. xx, fig. 19 (Anchitherium (?) australe); p. 251-2, pl. vii, figs. 16, 17 (Anchitherium agreste) Leidy, J.T. 1883. On remains of horses. Proc. Acad. Nat. Sci. Phila. 34:290-291, text-fig. p. 291 (Hippotherium montezuma) Leidy, J.T. 1885. Rhinoceros and Hippotherium from Florida. Proc. Acad. Nat. Sci. Phila., 37:33, text-fig. p. 33 (Hippotherium ingenuum) Leidy, J.T. 1887. Fossil bones from Florida, Proc. Acad. Nat. Sci. Phila. 39:309-310, text-fig. p. 310 (Hippotherium plicatile) Leidy, J.T. 1890. Hippotherium and Rhinoceros from Florida. Proc. Acad. Nat. Sci. Phila., 42:182-3, text-fig. p. 182 (Equus princeps) Lewin, R. 1980. Evolutionary theory under fire. Science 210:883-887. Lindsay, E.H., N.M. Johnson, and N.D. Opdyke. 1975. Preliminary correlation of North American Land Mammal Ages and geomagnetic chronology, in G.R. Smith and N.E. Friedland, eds., Studies in Cenozoic Paleontology and Stratigraphy. Univ. Michigan Pap. Paleontol. No. 12, pp. 111-119. Lindsay, E.H., N.M. Johnson, N.D. Opdyke, and R.F. Butler. 1987. Mammalian chronology and the magnetic polarity time scale, in M.O. Woodburne, ed., Cenozoic Mammals of North America: Geochronology and Biostratigraphy. The University of California Press, Berkeley, pp. 267-284. Lindsay, E.H., N.D. Opdyke and N.M. Johnson. 1980. Pliocene dispersal of the horse Equus and late Cenozoic mammalian dispersal events. Nature 287:135-138. Linnaeus, Carolus (Karl Linné). 1758. Systema Naturae, per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species cum Chatracteribus, Differentiis Synonymis, Locus. Editio Decima. Salvii, [Stock]Holmiae, 1:1-824. Loomis, F.B. 1908. A new horse from the lower Miocene. Amer. Jour. Sci. and Arts, 26(152):163-4, fig. 1.(Parahippus tyleri). Lundelius, E.L. Jr. T. Downs, E.H. Lindsay, H.A. Semken, R.J. Zakrzewski, C.S. Churcher, C.R. Harington, G.E. Schultz, and S.D. Webb. 1987. The North American Quaternary sequence, in M.O. Woodburne, Cenozoic Mammals of North America: Geochronology and Biostratigraphy. The University of California Press, Berkeley, pp. 211-235. Lundelius, E.L. Jr. and M.S. Stevens. 1970. Equus francisi Hay, a small stiltlegged horse, middle Pleistocene of Texas. Journal of Paleontology 44(1):148-153. Lundholm, B. 1949. Abstammung und domestikation des Hauspferdes. Zoologiska Bidrag fran Uppsala 27:1-269. Lydekker, R. 1896. A Geographical History of Mammals. Cambridge University Press. Macarovici, N. 1967. Kritischer Uberblick über Hipparion im Neogen von Rumanien. Osterr. Akad. Wiss., Math. Naturw. Kl., Sitzber., Abt. I, 176(5-7). Marsh, O.C. 1868. Notice of a new and diminutive species of fossil horse (Equus parvulus), from the Tertiary of Nebraska. Amer. Jour. Sci. and Arts 46(138):374-5, no fig. (Equus parvulus) Marsh, O.C. 1874. Notice of new Equine mammals from the Tertiary Formation. Amer. Jour. Sci. and Arts 7 (39):250, no fig. (Anchitherium anceps); 249, no fig. (Miohippus annectens, gen. et sp. nov.); pp. 253-4, no fig. (Protohippus avus); p. 254-55, no fig. (Anchippus brevidens); p. 252-3 (Pliohippus gen. nov.); p. 252-3, no fig. (Pliohippus pernix, gen. et sp. nov.); p. 253, no fig. (Pliohippus robustus) Marsh, O.C. 1892. Recent polydactyle horses. Amer. Jour. Sci. and Arts, 43(256):347, no fig. (Pliohippus gracilis). Martin, L.D., and D.K. Bennett. 1977. The burrows of the Miocene beaver Paleocastor, Western Nebraska, U.S.A. Palaeogeography, Palaeoclimatology, Palaeoecology, 22:173193. Matthew, W.D. 1897. A revision of the Puerco fauna. Bull. Amer. Mus. Nat. Hist. 9(22):259-323. Matthew, W.D. 1915. Climate and Evolution. Ann. New York Acad. Sci. 24:171-318. Matthew, W.D. 1926. The evolution of the horse. Quarterly Review of Biology, 1:130-185. Matthew, W.D. 1929. Critical observations upon Siwalik mammals. Bull. Amer. Mus. Nat. Hist. 56:437-560. Matthew, W.D., and R.A. Stirton. 1930. Equidae from the Pliocene of Texas. Univ. Calif. Publ., Bull. Dept. Geol. Sci. 19(17):349-396. Matthew, W.D., and J.W. Gidley. 1906. New or little known mammals from the Miocene of South Dakota. Bull. Amer. Mus. Nat. Hist. 22(7):151, figs. 18, 19, 20 (Neohipparion niobrarense); p. 139, no fig. (Protohippus simus); p. 143, figs. 8,9 (desig. neotype of Protohippus supremus Leidy); p. 148-49, fig. 14 (Neohipparion dolichops) MacFadden, B.J. 1976. Cladistic analysis of primitive equids with notes on other Perissodactyls. Syst. Zool. 25(1):1-14. MacFadden, B.J. 1980. The Miocene horse Hipparion from North America and from the type locality in southern France. Paleontology 23:617-634. MacFadden, B.J. 1984. Systematics and phylogeny of Hipparion, Neohipparion, Nannippus and Cormohipparion (Mammalia, Equidae) from the Miocene and Pliocene of the New World. Bull. Amer. Mus. Nat. Hist., 179:1-196. MacFadden, B.J. 1985. Patterns of phylogeny and rates of evolution in fossil horses: hipparions from the Miocene and Pliocene of North America. Paleobiology, 11:245-257. MacFadden, B.J. 1992. Fossil Horses: Systematics, Paleobiology and Evolution of the Family Equidae. Cambridge Univ. Press, new York, 369 pp. MacFadden, B.J. and M.F. Skinner. 1979. Diversification and biogeography of the onetoed horses Onohippidium and Hippidion. Yale Peabody Mus. Nat. Hist. Postilla 175:1-9. MacFadden, B.J. and M.F. Skinner. 1981. Earliest Holarctic hipparion, Cormohipparion goorisi n. sp. (Mammalia, Equidae), from the Barstovian (medial Miocene) Texas Gulf Coastal Plain. Jour. Paleontol. 55:619-627. MacFadden, B.J. and M.O. Woodburne. 1982. Systematics of the Neogene Siwalik hipparions (Mammalia, Equidae) based on cranial and dental morphology. Jour. Vert. Paleo. 2:185-218. MacFadden, B.J. and A. Azzaroli. 1987. Cranium of Equus insulatus (Mammalia, Equidae) from the middle Pleistocene of Tarija, Bolivia. Jour. Vert. Paleontol. 7:230-235. MacFadden, B.J. and R.C. Hulbert, Jr. 1988. Explosive speciation at the base of the adaptive radiation of Miocene grazing horses. Nature 336:466-468. MacFadden, B.J., J.D. Bryant, and P.A. Mueller. 1991. Sr-isotopic, paleomagnetic, and biostratigraphic calibration of horse evolution: evidence from the Miocene of Florida. Geology 19:242-245. Markova, A.K., N.G. Smirnov, A.V. Kozharinov, N.E. Kazantseva, A.N. Simakova, and L.M. Kitaev. 1995. Late Pleistocene distribution and diversity of mammals in northern Eurasia. Paleontolgia I Evolucio, 28-29:5-143. Marsh, Othniel Charles. 1876. Notice of new Tertiary mammals. Amer. Jour. Sci. 12:401-404. Marsh, O.C. 1879. Polydactyle horses, recent and extinct. Amer. Jour. Sci. 17:499-505. Marsh, O.C. 1884. Dinocerata: a monograph of an extinct order of gigantic mammals. U.S. Geol. Sur. Monographs 10:1-237. Martin, L.D. and A. M. Neuner. 1978. The end of the Pleistocene in North America. Trans. Nebraska Acad. Sci., 6:117-126. Martin, P.S. and R.G. Klein, eds. 1984. Quaternary Extinctions: A Prehistoric Revolution. The University of Arizona Press, Tucson. Martin, P.S. and H.E. Wright, Jr., eds. 1967. Pleistocene Extinctions: The Search for a Cause. Yale University Press, New Haven. Matthew, W.D. 1903. The evolution of the horse. Amer. Mus. Nat. Hist., Suppl. Amer. Mus. J. Guide Leaflet 9(3):1-30. Matthew, W.D. 1924. Third contribution to the Snake Creek Fauna. Bull. Amer. Mus. Nat. Hist. 50:59-210. Matthew, W.D. 1926. The evolution of the horse: a record and its interpretation. Quart. Rev. Biol. 1:139-185. Matthew, W.D. 1939. Climate and Evolution, 2nd ed. New York Academy of Sciences, Special Publication no. 1., New York. Mayr, Ernst O. 1969. Principles of Systematic Zoology. McGraw-Hill Book Co., New York. Mayr, Ernst O. 1970. Populations, Species, and Evolution. The Belknap Press, Harvard University, Cambridge, Massachussets. McGrew, P.O. 1938. The Burge Fauna, a lower Pliocene mammalian assemblage from Nebraska. Univ. Calif. Publ. Bull. Dept. Geol. Sci. 24:309-328. McGrew, P.O. 1944. An early Pleistocene (Blancan) fauna from Nebraska. Field Mus. Nat. Hist. Geol., 9:33-66. McGrew, P.O. 1953. A new and primitive early Oligocene horse from Trans-Pecos Texas. Fieldiana, Geol. 10:167-171. McGrew, P.O. 1971. Early Tertiary vertebrate faunas, Vieja group, Trans-Pecos Texas: Equidae. Mesohippus from the Vieja Group, Trans-Pecos Texas. Pearce-Sellards Series, Texas Mem. Mus., 18:6-11. McKenna, M.C. 1960. Fossil Mammalia from the early Wasatchian Four Mile Fauna, Eocene of northwest Colorado. Univ. Calif. Publ. Geol. Sci. 37:1-130. McKenna, M.C. 1972. Was Europe connected directly to North America prior to the middle Eocene? In T. Dobzhansky, M. Hecht and W.C. Steere, eds., Evolutionary Biology. Appleton-Century-Crofts, New York, pp. 179-188. McKenna, M.C. 1973. Sweepstakes, filters, corridors, Noah’s arks, and beached Viking funeral ships in paleogeography, in D.H. Tarling and S.K. Runcorn, eds., Implications of Continental Drift to the Earth Sciences, Vol. I. Academic Press, London, pp. 295-308. McKenna, M.C. 1975. Toward a phylogenetic classification of the Mammalia, in W.P. Luckett and F.S. Szalay, eds., Phylogeny of the Primates. Plenum Press, New York, pp. 21-46. McKenna, M.C. 1983. Cenozoic paleogeography of North Atlantic land bridges, in M.H.P. Bott, S. Savox, M. Talwani and J. Thiede, eds., Structure and Development of the Greenland-Scotland Ridge. Plenum Press, New York, pp. 351-399. McKenna, M.C. 1987. Molecular and morphological analysis of high-level mammalian interrelationships, in C. Patterson, ed., Molecules and Morphology in Evolution: Conflict or Compromise? Cambridge University Press, Cambridge, Massachussets, pp. 55-93. McKenna, M.C., M. Chow, S. Ting, and L. Zhexi. 1989. Radinskya yupingae, a perissodactyl-like mammal from the late Paleocene of China, in D.R. Prothero and R.M. Schoch, eds., The Evolution of Perissodactyls. Oxford University Press, pp. 24-36. McMahon, T.A. 1975. Allometry and biomechanics: limb bones in adult ungulates. Amer. Nat., 109:547-563. McNaughton, S.J., J.L. Tarrants, M.M. McNaughton, and R.H. Davis. 1985. Silica as a defense against herbivory and a growth promoter in African grasses. Ecology 66:528535. Merriam, J.C. 1913a. New protohippine horses from Tertiary beds on the western border of the Mohave Desert. Univ. Calif. Publ. Bull Dept. Geol. Sci., 7(23):440-441, figs. 4a, 4b (Pliohippus (?) tantalus) Merriam, J.C. 1913b. New Anchitheriine horses from the Tertiary of the Great Basin area. Univ. Calif. Publ. Bull. Dept. Geol. Sci., 7(22):420, 422, figs.1a,b, 2a,b (Hypohippus (Drymohippus) nevadensis); p. 427, fig. 3 (Parahippus (?) mourningi) Merriam, J.C. 1914. Correlation between the Tertiary of the Great Basion and that of the marginal, marine province in California. Science, N.S., 15(1035):644, no fig. (Protohippus coalingensis) Merriam, J.C. 1915a. New horses from the Miocene and Pliocene of California. Univ. Calif. Publ. Bull. Dept. Geol. Sci. 9(4):49-50, fig. 1 (Merychippus sumani); p. 50-52, fig. 2,3 (Merychippus intermontanus); p. 52, figs. 4a,b,c (Protohippus tehonensis); p. 55-56, figs. 8a,b,c (Pliohippus fairbanksi); p. 54-55, figs. 5-7 (Hipparion mohavense callodonte) Merriam, J.C. 1915b. Tertiary vertebrate faunas of the North Coalinga Region of California, a contribution to the study of palaeontologic correlation in the Great Basin and Pacific Coast Provinces. Trans. Amer. Philos. Soc., N.S. 22(3):3a,b,c,d. (Merychippus californicus) Merriam, J.C. 1915c. New species of the Hipparion group from the Pacific Coast and Great Basin provinces of North America. Univ. Calif. Publ., Bull. Dept. Geol. Sci. 9(1):6, fig. 4 (Hipparion condoni); p. 1-2, fig. 1 (Hipparion gidleyi); p. 5 (Neohipparion platystyle); p. 3, fig. 2 (Neohipparion molle); p. 3-4, fig. 3 (Neohipparion leptode) Merriam, J.C. 1915d. Vertebrate fauna of the Orindan and Siestan beds in middle California. Univ. Calif. Publ. Bull. Dept. Geol. Sci., 7(19):375-6, figs. 1a,b,c (Neohipparion sp.)(this is the original ref; see 1915c) Merriam, J.C. 1916a. Relationship of Equus to Pliohippus suggested by characters of a new species from the Pliocene of California. Univ. Cal. Publ. Bull. Dept. Geol. Sci., 9(18):525-534, figs. 2, 13 (Pliohippus proversus) Merriam, J.C. 1916b. Mammalian remains from the Chanac Formation of the Tejon Hills, California. Univ. Calif. Publ., Bull. Dept. Geol. Sci. 10(8):118-124, figs. 1a,b,c (Hipparion gratum tehonense) Merriam, J.C. 1916c. Mammalian remains from a late Tertiary formation at Ironside, Oregon. Univ. Calif. Publ., Bull. Dept. Geol. Sci., 19(9):131-133, figs. 1a,b,c (Hipparion anthonyi). Merriam, J.C. 1918. New Mammalia from the Idaho Formation. Univ. Calif. Publ., Bull. Dept. Geol. Sci. 10:523-530. Mones, A. 1982. An equivocal nomenclature: What means hypsodonty? Palaeontol. Zeitschrift 56:107-111. Moody, P.A. 1970. Introduction to Evolution, 3rd ed. Harper & Row, New York. Mooser, O., and W.W. Dalquest. 1975. Pleistocene mammals from Aguascalientes, Mexico. Jour. Mamm. 56(4):781-820. Moreau, R.E. 1955. Ecological changes in the Palaearctic region since the Pliocene. Proc. Zool. Soc. London, 125:253-295. Morlan, r.E. and J. Cinq-Mars. 1982. Ancient Beringians: human occupation in the late Pleistocene of Alaska and the Yukon Territory, in D.M. Hopkins, J.V. Matthews Jr., C.E. Schweger, and S.B. Young, eds., Paleoecology of Beringia. Academic Press, New York, 489 pp. Morris, W.J. 1968. A new early Tertiary Perissodactyl, Hyracotherium seekinsi, from Baja California. Los Angeles Co. Mus. Nat. Hist. Contrib. Sci. 151:1-11. Nehring, A. 1883. Uber neue bei Westeregeln gemachte Fossilfunde, sowie uber die Vorgeschichte des Pferdes in Europa. S.B. Ges. naturf. Fr. Berlin. Nelson, G., and N. Platnick. 1981. Systematics and Biogeography: Cladistics and Vicariance. Columbia University Press, New York. Nelson, G., and D.E. Rosen, eds. 1981. Vicariance Biogeography: A Critique. Columbia University Press, New York. Neville, C., N.D. Opdyke, E.H. Lindsay, and N.M. Johnson. 1979. Magnetic stratigraphy of Pliocene deposits of the Glenns Ferry Formation, Idaho, and its implications for North American mammalian biostratigraphy. Amer. Jour. Sci. 279:503-526. Nicholson, T.D., B. Schaeffer, T. Galusha, M.C. McKenna, M.F. Skinner, B.E. Taylor, and R.H. Tedford. 1975. The fossil mammal collections of the American Museum of Natural History. Curator 18:18-38. Novacek, M.J. 1982. The skull of leptictid insectivores and the higher-level classification of Eutherian mammals. Bull. Amer. Mus. Nat. Hist. 183:1-112. Novacek, M.J., A. Wyss, and M.C. McKenna. 1988. The major grouops of eutherian mammals, in M.J. Benton, ed., The Phylogeny and Classification of the Tetrapods, Vol. 2: Mammals. Systematics Association Special Volume no. 35B, Clarendon Press, Oxford, pp. 31-71. Olson, E.C. 1966. Community evolution and the origin of mammals. Ecology 47:291302. Opdyke, N.D. 1990. Magnetic stratigraphy of Cenozoic terrestrial sediments and mammalian dispersal. Jour. Geol., 98:621-637. Osborn, H.F. 1902. Dolichocephaly and brachycephaly in the lower mammals. Bull. Amer. Mus. Nat. Hist. 16:77-89. Osborn, H.F. 1908. From the Greeks to Darwin: An Outline of the Development of the Evolution Idea. MacMillan & Co., New York. Osborn, H.F. 1910. The Age of Mammals: In Europe, Asia, and North America. MacMillan & Co., New York. Osborn, H.F. 1904. New Oligocene horses. Bull. Amer. Mus. Nat. Hist. 20 (13):170 (Mesohippus montanensis); 173, fig. 4, pl.v, C. (M. obliquidens); 173-4, fig. 5 (M. eulophus); 167-179, figs. 1-8, pl. v., and pp. 174-175, pl. v., D (Mesohippus meteulophus); 175-6, fig. 6, Pl. v, E. (Mesohippus brachystylus); 177, pl. v, H (Mesohippus validus); p. 178, fig. 8, pl. v, G. (M. gidleyi); 178-9, no fig (Miohippus crassicuspis); p. 76, pl. 6.5, 9.7, 25.1, 36.1, text-fig. 52 (Parahippus pristinus) Osborn, H.F. 1912. Craniometry of the Equidae. Mem. Amer. Mus. Nat. Hist., new ser., 1(33):57-100. Osborn, H.F. 1915. [no title; description of Kalobatippus gen. Nov.], in E.D. Cope and W.D. Matthew, eds., Hitherto Unpublished Plates of Tertiary Mammalia and Permian Vertebrata, Amer. Mus. Nat. Hist. Monograph Ser. 2, plate CVIII. Osborn, H.F. 1917. The Origin and Evolution of Life: On the Theory of Action, Reaction and Interaction of Energy. Charles Scribner’s Sons, New York. Osborn, H.F. 1918. Equidae of the Oligocene, Miocene, and Pliocene of North America: iconographic type revision. Mem. Amer. Mus. Nat. Hist., New Ser., 2:1-217. (Mesohippus trigonostylus, p. 47, plate 2,5,6; text-fig. 27); p. 61, plate 5.1 (Miohippus primus); p. 62, plats 3.8, 4.6, 4.8, 5.2, text-figs. 40, 41 (Miohippus quartus); 65, plate 3.6, text-fig 45 (Miohippus equinanus); 66, plates 3.7, 4.l-4, 4.9, text-figs. 46-7 (Miohippus gemmarosae); p. 71, plates 5.7, 39.17, 39.20, 40, 51.1, 51.4; text-fig. 50 (Kalobatippus agatensis); p. 79, pl. 38, 39.9, 39.13, text-fig. 56 (Parahippus pawniensis); p. 83, pl. 8.1, 9.1, 36.2-3, text-fig. 58 (Parahippus coloradensis); p. 105, pl. 13.4, 41.1, 45.1-3, 46.1, 52.1, text-fig. 79 (Merychippus isonesus); p. 114, pl. 20.1, 39.6, 44.2, 47.1, 49.2, 54.2, text-fig. 90 (Merychippus eoplacidus); p. 117, pl. 11.3, 15.6, 15.7, 20.3, 54.1, text-fig. 91 (Merychippus proparvulus); p. 117, pl. 20.2, 44.3, 47.3, 50.4, 50.8, 54.3, text-fig. 92 (Merychippus eohipparion); p.125, pl. 10.1, 17.7, text-fig. 99 (Merychippus republicanus); p. 126, pl. 17.6, text-fig. 100 (Merychippus patruus); p. 139, pl. 25.7, 34.3, text-fig. 112 (Protohippus proplacidus); p. 144, p.ate 22.1, text-fig. 116, 116a (Protohippus perditus); p. 160, pl. 26.4, 28.4, text-fig. 128 (Pliohippus nobilis); p. 162, pl. 28.3, 29, 30.1, 30.2, text-fig. 129 (Pliohippus leidyanus); p. 183, text-fig. 146 (Hipparion coloradense) Osborn, H.F. 1934. Aristogenesis, the creative principle in the origin of species. Amer. Nat. 68:193-235. Osborn, H.F., and J.L. Wortman. 1895. Perissodactyls of the Lower Miocene White River Beds. Bull. Amer. Mus. Nat. Hist. 7(12):355, fig. 4 (Mesohippus intermedius) Osborn, H.F. and W.D. Matthew. 1909. Cenozoic mammal horizons of western North America. U.S. Geol. Surv. Bull. 361:1-138. Owen, R. 1840a. Description of the fossil remains of a mammal, a bird, and a serpent, from the London Clay. Proc. Geol. Soc. London 3:162-166. Owen, R. 1840b. The Zoology of the Voyage of the H.M.S. Beagle, Part 1: Mammalia. Smith Elder & Co., London Owen, R. 1848. Description of teeth and portions of jaws of two extinct anthracotheroid quadrapeds (Hyopotamus vectianus and H. bovinus) discovered by the Marchioness of Hastings in the Eocene deposits on the N.W. coast of the Isle of Wight, with an attempt to develop Cuvier’s idea of the classification of pachyderms by the number of their toes. Quart. Jour. Geol. Soc. London 4:104-141. Owen, R. 1868. On the Anatomy of Vertebrates. Vol. 3, Mammals. Longmans, Green & Co., London. Owen-Smith, N. 1985. Niche separation among African ungulates, in E.S. Vrba, ed., Species and Speciation. Transvaal Museum Monograph no. 4, Praetoria, pp. 167-171. Patterson, B. and R. Pascual. 1972. The fossil mammal fauna of South America, in A. Keast, F.C. Erk and B. Glass, eds., Evolution, Mammals, and Southern Continents. State Univ. New York Press, Albany. Peterson, O.A. 1907. The Miocene beds of western Nebraska and eastern Wyoming and their vertebrate faunae. Ann. Carneg. Mus., 4(11):57-60, pl. xix. (Parahippus nebrascensis). Pirlot, P.L. 1952. Les canines chez Hipparion et L’apparition D’un caractère sexual secondaire des mammifères. Bull. Mus. (Nat. Hist.), 2nd Ser. 24:419-422. Pirlot, P.L. 1956. Les formes européennes du genre Hipparion. Mem. y Com. Inst. Geol. Barcelona 14:1-222. Plotnick, R.E. 1986. A fractal model for the distribution of stratigraphic hiatuses. Jour. Geol. 94:885-890. Pomel, A. 1853. Catalogue methodique et descriptif des Vertebres fossiles du bassin hydrographique superieur de la Loire. Paris. Pomel, A. 1897. Monographies des Vertebres fossiles de l'Algerie. 10. Les Equides. Publ. Serv. Carte Geol. Algerie, Alger. Prat, F. 1980. Les Equidés Villafranchiens en France, genre Equus. Cahiers du Quaternaire, Centre National de la Recherche Scientifique. No. 2:1-290. Pratt, A.E. 1990. Taphonomy of the large vertebrate fauna from the thomas Farm locality (Miocene, Hemingfordian), Gilchrist County, Florida. Bull. Florida Mus. Nat. Hist., Biol. Sci. 35:35-130. Prothero, D.M., E.M. Manning, and M. Fischer. 1989. The phylogeny of ungulates, in M.E. Benton, ed., The Phylogeny and Classification of the Tetrapods, Vol. 2, Mammals. Clarendon Press, Oxford, pp. 201-234. Prothero, D.R. and N. Shubin 1989. The evolution of mid-Oligocene horses, in D.R. Prothero and R.M. Schoch, eds., The Evolution of Perissodactyls. Clarendon Press, Oxford, pp. 142-175. Prothero, D.R. and R.M. Schoch. 1989. Classification of the Perissodactyla, in D.R. Prothero and R.M. Schoch, eds., The Evolution of Perissodactyls. Clarendon Press, Oxford, pp. 504-529. Prout, H.A. 1847. Description of a fossil maxillary bone of a Palaeotherium from near White River. Amer. Jour. Sci. 3:248-50. Qiu, Z., W. Huang, and Z. Guo. 1987. The Chinese hipparionine fossils. Palaeont. Sinica, Ser. C, 25:1-250. Quinn, J.H. 1955. Miocene Equidae of the Texas Gulf Coastal Plain. Univ. Tex. Bur. Econ. Geol. Publ. 5516:1-102. Quinn, J.H. 1957. Pleistocene Equidae of Texas. University of Texas Bureau of Economic Geology Rept. Invest. no. 33:1-51. Radinsky, L.B. 1966. The adaptive radiation of the phenacodontid condylarths and the origin of the Perissodactyla. Evolution 20(3):408-417. Radinsky, L.B. 1969. The early evolution of the Perissodactyla. Evolution 23:308-326. Radinsky, L.B. 1976. Oldest horse brains: more advanced than previously realized. Science 194:626-627. Radinsky, L.B. 1978. Evolution of brain size in carnivores and ungulates. Amer. Nat. 112:815-831. Radinsky, L.B. 1983. Allometry and reorganization in horse skull proportions. Science 221:1189-1191. Radinsky, L.B. 1984. Ontogeny and phylogeny in horse skull evolution. Evolution, 38:115. Rau, R.E. 1986. The quagga and its kin. Sagittarius 1(2):8-10. Raup, D.M. 1975. Taxonomic survivorship curves and Van Valen’s law. Paleobiology 1:82-96. Reichenau, W. von. 1903. Über einen Unterkiefer von Equus stenonis Cocchi aus dem Pliopleistocan von Mosbach. Notizblatt des Grossh. Geologischen Landesanstalt zu Darmstadt, 4(24):48-54. Reichenau, W. von. 1915. Beitrage zur naheren Kenntnis fossiler Pferde aus deutschem Pleistozan. Abh. geol. Landesanst. Darmstadt. 7:1. Renders, E. 1984. The gait of Hipparion sp. From fossil footprints in Laetoli, Tanzania. Nature 308:179-181. Renders, E., and P.Y. Sondaar. 1987. Hipparion, in M.D. Leakey and J.M. Harris, eds., Laetoli: A Pliocene Site in Northern Tanzania. Clarendon Press, Oxford, pp. 471-481. Rensberger, J.M., A. Forstèn, and M. Fortelius. 1984. Functional evolution of the cheek tooth pattern and chewing direction in Tertiary horses. Paleobiology 10:439-452. Repenning, Charles A. 1967. Palearctic-Nearctic mammalian dispersal in the late Cenozoic, in d.M. Hopkins, ed., The Bering Land Bridge. Stanford Univ. Press, Stanford, California, pp. 288-311. Rémy, J.A. 1965. Un nouveau genre de Paleotheriidae (Perissodactyla) de l’Eocene supérior du Midi de la France. C.R. Acad. Sci. Paris, 20:4362-4364. Rémy, J.A. 1972a. Etude de crane de Pachynolophus lavocati n. sp. (Perissodactyla, Palaeotheriidae) des Phosphorites du Quercy. Palaeovertebvrata 5:45-78. Rémy, J.A. 1972b. Evolution d’une structure histologique chèz les perissodactyles: le development de la dentine pericanaliculaire. C.R. Acad. Sci. Paris, 274:2026-2029. Richey, K.A. 1948. Lower Pliocene horses from Black Hawk Ranch, Mount Diablo, California. Univ. Calif. Bull. Dept. Geol. Sci. 28:1-44. Romer, Alfred Sherwood. 1966. Vertebrate Paleontology. University of Chicago Press, Chicago, Illinois. Rose, K.D. 1990. Postcranial skeletal remains and adaptations in early Eocene mammals from the Willwood Formation, Bighorn Basin, Wyoming, in T.M. Brown and K.D. rose, eds., Dawn of the Age of Mammals in the Northern Part of the Rocky Mountain Interior, North America. Geol. Soc. Amer. Spec. Pap. No. 243, pp. 107-133. Rutimeyer, L. 1863. Beitrage zur Kenntniss der fossilen Pferde und zur vergleichenden Odontographie der Hufthiere uberhaupt. Verh. naturf. Ges. Basel 3. Sack, W.O. 1989. The stay-apparatus of the horse’s hind limb – explained. Equine Practice, 11:31-35. Samson, P. 1975. Les Equides fossiles de Roumanie (Pliocene moyen - Pleistocene superieur). Geologica Rom., 14(1975):165-352. Savage, D.E. 1951. Late Cenozoic vertebrates of the San Francisco Bay region. Univ. Calif. Publ. Dept. Geol. Sci. 28:215-314. Savage, D.E., D.E. Russell and P. Louis. 1965. European Eocene Equidae. Univ. Calif. Publ., Bull. Dept. Geol. Sci., 56:1-94. Savage, J.M. 1977. Evolution, 3rd ed. Holt, Rinehart & Winston, New York. Savage, R.J.G., and M.R. Long. 1986. Mammal Evolution: An Illustrated Guide. FactsOn-File Publications, New York. Schafer, T,.S., and W.W. Dalquest. 1991. Comparison of dental characters of fossil horses in two Pleistocene local faunas. Texas Jour. Sci., 43:45-48. Schaeffer, B. 1947. Notes on the origin and function of the artiodactyl tarsus. Amer. Mus. Novitates, 1356:1-24. Schaeffer, B., M.K. Hecht and Niles Eldredge. 1972. Phylogeny and paleontology, in T. Dlobzhansky, M.K. Hecht and W.C. Steered, eds., Evolutionary Biology, Vol. 6. Appleton-Century-Crofts, New York, pp. 31-46. Schiebout, J.A. 1974. Vertebrate Paleontology and paleoecology of the Paleocene Black Peaks Formation, Big Bend National Park, Texas. Texas Mem. Mus. Bull. 24:1-88. Schoch, R.M. 1989. A brief historical review of Perissodactyl classification, in D.R. Prothero and R.M. Schoch, eds., The Evolution of Perissodactyls. Clarendon Press, Oxford, pp. 13-23. Schuchert, C., and C.M. LeVene. 1940. O. C. Marsh, Pioneer in Paleontology. Yale University Press, New Haven, pp. 1-541. Schultz, J.R. 1936. Plesippus francescana (Frick) from the Late Pliocene, Coso Mountains, California, with a review of the genus Plesippus. Carneg. Inst. Wash. Publ. no. 473:1-13. Schwarzbach, M. 1963. Climates of the Past. Van Nostrand, London. Scott, E. 1990. New record of Equus conversidens Owen, 1869 (Mammalia; Perissodactyla; Equidae) from Rancho La Brea. Jour. Vert. Paleontol. 10:41A. Scott, William Berryman. 1893. The mammals of the Deep River Beds. Amer. Nat. 27 (319):661, no fig. (Desmatippus crenidens); p. 94, pl. iii, figs. 25-28 (Anchitherium equinum) Scott, W.B. 1894. The Mammalia of the Deep River Beds. Trans. Amer. Philos. Soc., N.S. 28 (1894): 84, pl. ii, figs. 9-14 (more on Desmatippus crenidens). Scott, W.B. 1913. A History of Land Mammals in the Western Hemisphere. MacMillan, New York. Scott, W.B. 1941. The mammalian fauna of the White River Oligocene, Part V: Perissodactyla. Trans. Am. Philos. Soc. 28:747-980, plates 79-100. Sefve, I. 1912. Die fossilen Pferde Südamerikas. Kungl. Svenska Vetenskapsakademiens Handlingar, 48:1-185. Sefve, I. 1927. Die Hipparionen Nord-Chinas. Paleont. Sinica Ser. C, Vol. 4, Fasc. 22:1-91. Sellards, E.H. 1916. Fossil vertebrates from Florida: a new Miocene fauna; new Pliocene species; the Pleistocene fauna. Eighth Ann. Rept. Florida State Geol. Surv., pp. 83-7, pl. 11, fig. 7, Pl. 13, figs. 2,3 (Parahippus leonensis); p. 96-98, pl. 11, fig. 10, pl. 13, fig. 8 (Hipparion minor) Shikama, T., and Y. Onuki. 1962. Equid fossils from Iwate and Miyagi prefectures. Science Reports, Tohuku Universkity (Sendai, Japan), Geology, 34:187-197. Short, R.V. 1975. The evolution of the horse. Jour. Reproduction and Fertility, 23:1-6. Shotwell, J.A. 1961. Late Tertiary biogeography of horses in the northern Great Basin. Jour. Paleontol. 35:203-217. Simpson, George Gaylord. 1933. Glossary and correlation charts of North American Tertiary mammal-bearing formations. Bull. Amer. Mus. Nat. Hist. 67:79-121. Simpson, G. G. 1945. The principles of classification and a classification of mammals. Bull. Amer. Mus. Nat. Hist., 85:10-350. Simpson, G.G. 1940. Mammals and land bridges. Jour. Wash. Acad. Sci. 30:137-163. Simpson, G.G. 1944. Tempo and Mode in Evolution. Columbia University Press, New York. Simpson, G.G. 1945. The principles of classification and a classification of mammals. Bull. Amer. Mus. Nat. Hist. 85:1-350. Simpson, G.G. 1951. Horses: The Story of the Horse Family in the Modern World and Through Sixty Million Years of History. Oxford University Press, New York. Simpson, G.G. 1952. Notes on British hyracotheres. Jour. Linn. Soc. London, Zool., 42(284):195-206. Simpson, G.G. 1953. The Major Features of Evolution. Columbia University Press, New York. Sinclair, W.J. 1905. New or imperfectly known rodents and ungulates from the John Day series. Univ. Calif. Publ., Bull. Dept. Geol. Sci., 4(6):141, pl. xviii (Mesohippus acutidens). Skinner, Morris F. 1972. Order Perissodactyla, in: M.F. Skinner and C.W. Hibbard et al., eds., Early Pleistocene preglacial and glacial rocks and faunas of North-Central Nebraska. Bull. Amer. Mus. Nat. Hist. 148(1). Skinner, Morris F., and B.E. Taylor. 1967. A revision of the geology and paleontology of the Bijou Hills, South akota. Amer. Mus. Novitates 2300:1-53. Skinner, Morris F. and C.W. Hibbard. 1972. Early Pleistocene pre-glacial and glacial rocks and faunas of north-central Nebraska. Bull. Amer. Mus. Nat. Hist., 148:1-148. Skinner, Morris F. and F.W. Johnson. 1984. Tertiary stratigraphy and the Frick collection of fossil vertebrates from north-central Nebraska. Bull. Amer. Mus. Nat. Hist., 178:215368. Skinner, Morris F. and B.J. MacFadden. 1977. Cormohipparion n. gen. (Mammalia, Equidae) from the North American Miocene (Barstovian, Clarendonian). Jouir. Paleo., 51:912-926. Skinner, Morris F., S.M. Skinner, and R.J. Gooris. 1977. Stratigraphy and biostratigraphy of Late Cenozoic deposits in central Sioux County, western Nebraska. Bull. Amer. Mus. Nat. Hist., 158:263-370. Slijper, E.J. 1946. Comparative biologic-anatomical investigations on the vertebral column and spinal musculature of mammals. Verh. Onink. Nederl. Akad. Wetensch. Afd. Natuurk., 42:1-128. Skorkowski, E. 1938. Studien uber die Systematik des Pferdes. Pol. Akad. Umiejetnosci. 32:1-125. Smith, A.B. and C. Patterson. 1988. The influence of taxonomic method on the perception of patterns of evolution, in M.K. Hecht and B. Wallace, eds., Evolutionary Biology, Vol. 23. Plenum Publishing, New York, pp. 127-216. Smith, C.H. 1845. Mammalia; Horses in W. Jardine, ed., The Naturalist’s Library. W.H. Lizars, Edinburgh, 20:1-352. Smith, J.M. and R.J.G. Savage. 1959. The mechanics of mammalian jaws. School Sci. Rev., 141:289-301. Sondaar, P.Y. 1968. The osteology of the manus of fossil and recent equidae with special reference to phylogeny and function. Verhandelingen der koniklijke nederlandse akademie van wetenschappen, afd. Naturkunde 24(1):1-76. Speed, J.G. and M.G. Etherington. 1952/1953. The Exmoor pony, and a survey of the evolution of horses in Britain. British Veterinary Journal, 108:229-338 and 109:315-320. Stanley, S.M. 1974. Relative growth of the titanothere horn: a new approach to an old problem. Evolution 28:447-457. Stanley, S.M. 1979. Macroevolution: Pattern and Process. Freeman & Co., San Francisco. Stanley, S.M. 1981. The New Evolutionary Timetable: Fossils, Genes, and the Origin of Species. Basic Books, New York. Stehlin, H.G. and P. Graziosi. 1935. Recherchi sugli Assinidi fossili d'Europa. Mem. Soc. Pal. Suisse 56. Stirton, R.A. 1940. Phylogeny of North American Equidae. Univ. Calif. Publ., Bull. Dept. Geol. Sci., 25:165-198. Stirton, R.A. 1941. Development of characters in horse teeth and the dental nomenclature. Jour. Mamm., 22:339-410. Stirton, R.A. 1947. Observations on evolutionary rates in hypsodonty. Evolution 1:32-41. Tarling, D.H. 1983. Palaeomagnetism: Principles and Applications in Geology, Geophysics, and Archaeology. Chapman & Hall, London. Tedford, R.H. 1970. Principles and practices of mammalian geochronology in North America. Proc. 3rd North Amer. Paleontol. Convention F:666-703. Tedford, R.H., T. Galusha, M.F. Skinner, B.E. Taylor, R.W. fields, J.R. Macdonald, J.M. Rensberger, S.D. Webb and D.P. Whistler. 1987. Faunal succession and biochronology of the Arikareean through Hemphillian interval (late Oligocene through earliest Pliocene epochs), North America, in M.O. Woodburne, ed., Vertebrate Paleontology as a Discipline in Geochronology. Univ. Calif. Press, Berkeley. Thewissen, J.G.M. 1990. Evolution of Paleocene and Eocene Phenacodontidae (Mammalia, Condylarthra). Univ. Michigan Pap. Paleontol. 29:1-107. Thomason, J.J. 1985. The relationship of trabecular architecture to inferred loading patterns in the third metacarpals of the extinct equids Merychippus and Mesohippus. Paleobiology 11:232-325. Thomason, J.J. 1986. The functional morphology of the manus in the tridactyl equids Merychippus and Mesohippus: paleontological inferences from neontological models. Jour. Vert. Paleontol. 6:143-161. Thomason, J.J. 1992. Functional interpretation of locomotory adaptations during equid evolution. Soc. For Experimental Biol. Seminar Series, 36:213-227. Thomason, J.J., N.N. Biewener, and J.E.A. Bertram. 1992. Surface strain on the equine hoof wall in vivo: implications for the material design and functional morphology of the wall. Jour. Experimental biol., 166:145-168. Troxell, E. 1915. The vertebrate fossils of Rock Creek, Texas. Amer. Jour. Sci. 39:613638. Troxell, E. 1916. An early Pliocene one-toed horse, Pliohippus lullianus sp. nov. Amer. Jour. Sci., 42(250):335-348, figs. 1-7 (Pliohippus lullianus) Trumler, E. 1959. Das “Rossigkeitsgesicht” und ähnliches Ausdrucksverhalten bei Einhufern. Z. Tierpsychol. 16:478-488. Trumler, E. 1961. Entwurf einer systematik der rezenten Equiden ind ihrer fossilen Verwandten. Säugetierkundliche Mitteilungen, 9:109-125. Turnbull, P.FR. 1986. Measurements of Equus hemionus from Palegawra Cave (Zarzian, Iraq), in R.H. Meadows and H.-P. Uerpmann, eds., Equids in the Ancient World. L. Reichert Verlag, Wiesbaden, pp. 319-365. Uerpmann, H.-P. 1976. Equus (Equus) caballus und Equus (Asinus) hydruntinus im Postpleistozän der Iberischen Halbinsel (Perissodactyla, Mammalia). Säugetierkundlishce Mitteilungen, 24:206-218. Uerpmann, H.-P. 1987. The ancient distribution of ungulate mammals of the Middle East. Beihefte zum Tübinger Atlas des Vorderen Orients. Reihe A 9 (Naturwissenschaften). L. Reichert, Wiesbaden, 27:1-173. Van Valen, Lee. 1960. A functional index of hypsodonty. Evolution 14:531-532. Van Valen, Lee. 1963. Selection in natural populations: Merychippus primus, a fossil horse. Nature 197:1181-1183. Van Valen, Lee. 1964. Age in two fossil horse populations. Acta Zool. 45:93-106. Van Valen, Lee. 1965. Selection in natural populations. III. Measurement and estimation. Evolution 19:514-528. Van Valen, Lee. 1973. A new evolutionary law. Evol. Theory 1:1-30. Van Valen, Lee. 1978. Why not to be a cladist. Evol. Theory 3:285-299. Vaughan, T.A. 1972. Mammalogy. W.B. Saunders, Philadelphia. Vereshchagin, N.K. 1959. Mlekopitayushchie Kavkaza: istoriya formirovanniya fauny [The Mammals of the Caucasus: A history of the eevolution of the fauna]. Akademi Nauk., Moscow-Leningrad, pp. 1-704. Viret, J. 1954. Le loess a bancs durcis de Saint-Vallier (Drome) et sa fauna de Mammiferes villafranchiens. Nouv. Arch. Mus. Hist. Nat. Lyon, 4. Voorhies, M.R. 1969. Taphonomy and population dynamics of an early Pliocene vertebrate fauna, Knox County, Nebraska. Contrib. Geol., Univ. Wyoming, Spec. Pap. 1:1-69. Voorhies, M.R. 1981. Dwarfing the St. Helens eruption: ancient ashfall creates a Pompeii of prehistoric animals. Natl. Geogr. 159:66-75. Voorhies, M.R., and J.R. Thomasson. 1979. Fossil grass antothecia within Miocene rhinoceros skeletons: diet of an extinct species. Science 206:331-333. Voorhies, M.R. ---Vrba, E.S. and N. Eldredge. 1984. Individuals, hierarchies, and processes: towards a more complete evolutionary theory. Paleobiology 10:146-171. Wallace, A.R. 1876. The Geographical Distribution of Animals, Vol. 1. Harper and Brothers, New York. Webb, S. David. 1969a. The Burge and Minnechaduza Clarendonian mammalian faunas of north-central Nebraska. Univ. Calif. Publ. Bull. Dept. Geol. Sci., 78:1-191. Webb, S. D. 1969b. Extinction-origination equilibria in late Cenozoic land mammals of North America. Evolution 23:688-702. Webb, S.D. 1974. Chronology of Florida Pleistocene mammals, in S.D. Webb, ed., Pleistocene Mammals of Florida. University Presses of Florida, Gainesville, pp. 5-31. Webb, S.D. 1977. A history of savanna vertebrates in the New World, Part I: North America. Ann. Rev. Ecol. Syst. 8:355-380. Webb, S.D. 1978. A history of savanna vertebrates in the New World, Part II: South America and the Great Interchange. Ann. Rev. Ecol. Syst. 9:393-426. Webb, S.D. 1983. The rise and fall of the late Miocene ungulate fauna in North America, in M.H. Nitecki, ed., Coevolution. The University of Chicago Press, Chicago, pp. 267306. Webb, S.D. 1984. Ten milion years of mammal extinctions in North America, in P.S. Martin and R.G. Klein, eds., Quaternary Extinctions: A Prehistoric Revolution. The University of Arizona Press, Tucson, pp. 189-210. Webb, S.D., B.J. MacFadden, and J.A. Baskin. 1981. Geology and paleontology of the Love Bone Bed from the late Miocene of Florida. Amer. Jour. Sci. 281:513-544. Webb, S.D. and R,.C. Hulbert, Jr. 1986. Systematics and evolution of Paseudhipparion (Mammalia, Equidae) from the late Neogene of the Gulf Coastal Plain and the Great Plains. Univ. Wyoming Contrib. Geol., Spec. Paper no. 3, pp. 237-272. Webb, S.D., G.S. Morgan, R.C. Hulbert, Jr., D.S. Jones, B.J. MacFadden, and P.A. Mueller. 1989. Geochronology of a rich early Pleistocene vertebrate fauna, Leisey Shell Pit, Tampa Bay, Florida. Quat. Res. 32:96-110. West, R.M. 1976. The North American Phenacodontidae (Mammalia, Condylarthra). Conbrib. Biol. Geol., Milwaukee Publ. Mus. 6:1-78. White, T.E. 1959. The endocrine glands and evolution, no. 3: os cementum, hypsodonty, and diet. Univ. Mich. Mus. Paleont. Contrib., 13:211-265. Wiley, E.O. 1981. Phylogenetics: The Theory and Practice of Phylogenetic Systematics. Wiley & Sons, New York. Willoughby, D.P. 1974. The Empire of Equus. A.S. Barnes and Co., Philadelphia, pp. 1475. Winans, M. 1989. A quantitative study of North american fossil species of the genus Equus, in D.R. Prothero and R.M. Schoch, eds., The Evolution of Perissodactyls. Oxford University Press, New York, pp. 262-297. Wood, H.E. II. 1937. Perissodactyl suborders. Jour. Mammal. 18:106. Wood, H.E. II, R.W. chaney, J. Clark, E.H. Colbert, G.L. Jepsen, J.B. Reeside, Jr., and C. Stock. 1941. Nomenclature and correlation of the North American continental Tertiary. Bull. Geol. Soc. Amer. 52:1-48. Woodburne, M.O. 1987. Cenozoic Mammals of North America: Geochronology and Biostratigraphy. The University of California Press, Berkeley. Woodburne, M.O. and R.L. Bernor. 1980. On superspecific groups of some Old World hipparionine horses. Jour. Paleontol. 54:1919-1348. Woodburne, M.O., MacFadden, B.J. and M.F. Skinner. 1981. The North American "Hipparion" datum and implications for the Neogene of the Old World. Geobios 14(4):493-524. Wortman, J.L. 1882. On the origin and development of the existing horses. Kansas City Rev. Sci. and Industry, 6(2):73, no fig. (Hippotherium sinclairi). Wright, H.E. Jr. 1970. Vegetational history of the central plains, in W.E. Dort and J.K. Jones, eds., Pleistocene and Recent Environments of the Central Great Plains. Univ. Press. Kansas, pp. 157-172. Xiao Fang and Qiu Youxiang. 1990. [The skull of Equus prezewalskii]. Acta Theriologica sinica, 10:237-238 (in Chinese, English summary).