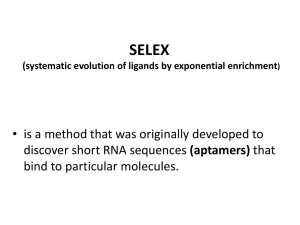
Castro_Martha_Gene Therapy HIV Techniques
advertisement

Castro 1 Martha Castro Dr. L Biomedical Cluster 7 27 July, 2015 Fighting HIV-1 Virus Using Fingers and TALENs There are thousands of diseases in our world today that stem from a flaw in genetic codes. Gene therapy seeks to fix those diseases by editing a person’s gene, whether it is through inserting, mutating, or deleting DNA. Zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) are two of the tools that are used in gene therapy to create double-stranded breaks in DNA. Both ZFNs and TALENs are used to target the CCR5 gene to create a 32 base pair deletion that prevents HIV from establishing and maintaining itself inside the human body. Gene therapy has emerged throughout the world as a “deliberate administration of genetic material into a human patient with the intention of correcting a specific genetic defect” (Office of Technology Assessment 2). Currently, this modification of DNA within a person’s body would not include “genetically enhancing general characteristics such as behavior, intelligence, or physical appearance” (Office of Technology Assessment 2). Gene therapy for now is limited to changing DNA with the intent only of fixing a genetic defect such as a mutation or deletion of a gene, which causes the person to contract a disease. In this paper, I will describe potential techniques using gene therapy for treating the HIV-1 virus. The gene editing techniques discussed in this paper, zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases TALENs, both induce double-stranded breaks in DNA, which are repaired by either non-homologous end joining (NHEJ) or homology-directed repair (HDR), two ways that DNA repairs itself in a cell (Yamamoto 3). “Homologous” refers to having the same origins and functions, so non-homologous end joining after a double-stranded break in DNA would mean that different parts of DNA would join together at the cleavage site. Castro 2 This would result in both interruptions and deletions of DNA around the area. Homologydirected repair can be thought of in a comparable way, except homologous DNA bond together to repair the break. This process makes for a precise joining together of DNA, which can be used to either edit the sequence of DNA within the cell or insert DNA from outside of the cell (Pellagatti 1-2). Both ZFNs and TALENs “have the advantage that they in principle can be designed to cleave nearly any site” (Humbert, Davis, and Maizels 268). ZFNs and TALENs are two of many techniques that genetic engineers use in gene therapy. Zinc Finger Nucleases (ZFNs): ZFNs are “artificial endonucleases that consist of a FokI cleavage domain tethered to a Cys2His2 zinc-finger” (Carlson, Fahrenkrug, and Hackett). FokI is a “type IIS restriction enzyme that cleaves DNA at a defined distance away from their recognition sites” (Yamamoto 6) while the Cys2His2 zinc-finger protein recognizes and contacts the DNA. Each ZFN is composed of about 30 amino acids that form a hydrophobic endonuclease in a ββα-fold that binds to three base pairs (Carlson, Fahrenkrug, and Hackett). Each ZFN can only bind to three base pairs each; therefore sections of 3-6 ZFNs are used to connect with 9-18 base pairs typically to form a zinc finger monomer (Carlson, Fahrenkrug, and Hackett). Each zinc finger contacts the three base pairs using the -1, 3, and 6 amino acids in the alpha helix. ZFNs are used in pairs on account of the dimerization that FokI nuclease domains require in order to create double strand breaks (Carlson, Fahrenkrug, and Hackett). “The two monomers of the ZFN pair must bind together in a tail-to-tail format, such that their C-termini (containing cleavage domains) face each other” (Mackay 26), creating two strands of ZFN monomers each comprised of 3-6 zinc fingers each. After ZFNs are constructed and inserted into a person’s genes, they can function in different ways to apply gene therapy to cells. ZFNs bind to DNA, which can inhibit viral Castro 3 transcription. One example that ZFNs can work on is the Hepatitis B virus, where they are able to bind to specific known sites of transcriptional regulation where the transcripts are known (Mackay 97). This method is useful for preventing viruses from reproducing, effectively stopping the progression of their disease. In addition, ZFNs can also be used to modulate gene expression, or turn genes on and off depending on the need. Modulating genes requires an artificial transcription factor that, at the very least, “consists of a sequence-specific DNA recognition module coupled to a transcriptional effector domain” (Mackay 117). ZFNs have also been used to “generate large DNA deletions, by simultaneous cleavage of a chromosome at two sites to promote deletion of the entire region between the breaks” (Humbert, Davis, and Maizels 271). They have successfully targeted and knocked out germ lines in genes by creating doublestrand breaks and NHEJ in animals, including zebra fish, mice, rats, rabbits, and pigs (Carlson, Fahrenkrug, and Hackett). “Gene delivery methods suitable to express ZFNs include plasmid DNA nucleofection, integrase-defective lentiviral vectors, and adenoviral vectors” (Holt 840). Gene therapy is made possible by using these vectors to insert ZFNs and other gene modifiers into the human body. Although ZFNs have “revolutionized the life science field” (Yamamoto 3), there are also some potential drawbacks to engaging in ZFN use for gene therapy. The usage of ZFNs has been associated with the risk of cytotoxicity, or the killing of cells. However, this occurs when cleavage in off-target sites happens during the dimerization process, which has been improved with a “structure based design of the FokI dimerization interface that blocks homodimerization” (Carlson, Fahrenkrug, and Hackett), which reduces the chance of cytotoxicity during gene therapy. Castro 4 Transcription Activator-like Effector Nucleases: Similarly to ZFNs, TALENs function to create double-stranded breaks in DNA in order that they might cure someone of a genetic disease. TALENs are made of amino acid repeats, 34 amino acids long (Carlson, Fahrenkrug, and Hackett). The 12th and 13th residues are called repeat variable residues and they function to recognize the specific base that the TALEN binds to (Carlson, Fahrenkrug, and Hackett). Inside an operative TALEN, there is a FokI nuclease, which cleaves the DNA, bonded to the C-terminus of the protein and a TAL effector binder core several spacer lengths away from the FokI nuclease (Carlson, Fahrenkrug, and Hackett). “Nuclear location signals and an acidic transcription-activation domain” (Carlson, Fahrenkrug, and Hackett) make up the TAL effector and allow it to bond to specified sequences of DNA. TAL effector proteins come from Xanthomonas, a bacterial plant pathogen, and other related plant genera that contain “an N-terminal domain, a DNA-binding domain, and a C-terminal domain including an activator domain” (Yamamoto 14). HIV Gene Therapy Treatment: HIV is a disease that targets T cells and reduces the number of them inside a person. The CCR5 gene is a major HIV co-receptor and allows the virus to dock in the body (Holt 839). There are individuals in the world who have a 32 base pair deletion in their CCR5 gene (Holt 839), which makes them resistant to infection through their CCR5 gene. One option to modify the CCR5 gene is by creating a 32 base pair deletion through cleavage in the cell. This technique was “demonstrated in an HIV-infected patient undergoing transplantation from a homozygous CCR5Δ32 donor during treatment for acute myeloid leukemia” (Holt 839). The patient received cells from a person who had the 32 base pair deletion in their CCR5 gene. The cells reproduced in the patient’s body and created more cells with the CCR5 disruption, eventually leading his Castro 5 cells to resist the HIV-1 virus. This caused the patient’s “CD4+ T cells to be restored” (Holt 839), effectively putting an end to both his HIV and AIDS. HIV Treatment with ZFNs: In an effort to replicate the results that occurred in this patient, where the CCR5 gene was interrupted and 32 base pairs were deleted to produce HIV-resistant cells, scientists introduced the HIV-1 virus to human CD34+ hematopoietic stem/progenitor cells (HSPCs) in mice (Holt 840). The control group received no treatment and the virus progressed as it would without any outside influences. In the experimental group of mice, ZFNs disrupted the CCR5 gene inside their human CD34+ HSPCs, causing a 32 base pair deletion. 4 weeks after being infected by HIV, the CD4+ T-cell levels in the ZFN-treated mice reached a normal level (Holt 843). HIV depletes the number of CD4+ T-cells in your body; therefore this increase boded well for the experiment. In intestinal samples taken 8 and 9 weeks after the initial infection, “viral levels [of HIV-1] in the ZFN-treated mice were 4 orders of magnitude lower than in the untreated controls” (Holt 843). This means that the virus in the ZFN-treated mice was rapidly decreasing when compared to the control group. “By the 10- and 12-week time points, HIV-1 RNA was undetectable in the ZFN-treated mice” (Holt 843), meaning the HIV virus was no longer actively reproducing within the ZFN-treated mice. The experiment ended with the ZFN-treated mice “giving rise to polyclonal multi-lineage progeny in which the CCR5 gene was permanently disrupted” (Holt 839). In other words, by using ZFNs to interrupt the CCR5 gene in the CD34+ HSPC cells, scientists created offspring from the cells that they modified. The CCR5 genes inside the offspring cells were similarly affected by the base pair deletion, rendering them HIV resistant. Castro 6 HIV Treatment with TALENs: TALENs are also useful in creating a base pair deletion in the CCR5 gene because both they and ZFNs have similar binding and cleaving parts. HIV has two different potential strategies, “a functional cure and a sterilizing cure” (Khalili 311). An example of a sterilizing cure is one that completely wipes the HIV virus from an individual. CCR5 impairment is “the most advanced approach for a functional cure for HIV-1 to date” (Khalili 311). In fact, “both TALENs and ZFNs induced ~45 % efficiency in disrupting the CCR5 gene, but TALENs induced much lower toxicity and off-target effects” (Khalili 312). Therefore, although the study cited earlier was about ZFNs, TALENs are, in fact, better to use when creating a base pair deletion inside a cell because they create less damage in the tissues surrounding the cleavage site. By using ZFNs and TALENs, scientists all around the world are hopeful that we might be able to defeat the HIV-1 virus, which has plagued us ever since the early 1980s. By creating double-strand breaks in the CCR5 gene, the HIV-1 virus no longer has a place to latch onto inside the body. Although this technique was first discovered when doctors were treating an HIV-1 positive man for leukemia, the method has also been tested on groups of mice with human cell grafts. Both ZFNs and TALENs have both a cleavage domain and a bonding domain, which allow them to accurately target and modify strands of DNA by creating double-strand breaks. The possibilities that these tools can grant us are endless. If our knowledge and skills in genetic engineering continue to progress in the fashion that they are now, the sky is the limit for our future world. Castro 7 Works Cited Carlson, Daniel F., Scott C. Fahrenkrug, and Perry B. Hackett. "Targeting DNA With Fingers and TALENs." Molecular Therapy — Nucleic Acids 1.1 (2012): n. pag. Web of Science. Web. 16 July 2015. Holt, Nathalia, Jianbin Wang, Kenneth Kim, Geoffrey Friedman, Xingchao Wang, Vanessa Taupin, Gay M. Crooks, Donald B. Kohn, Philip D. Gregory, Michael C. Holmes, and Paula M. Cannon. "Human Hematopoietic Stem/progenitor Cells Modified by Zincfinger Nucleases Targeted to CCR5 Control HIV-1 in Vivo."H Nature Biotechnology 28.8 (2010): 839-47. Web of Science. Web. 18 July 2015. Human Gene Therapy: Background Paper. Washington, D.C.: Congress of the United States, Office of Technology Assessment, 1984. Print. Humbert, Olivier. "Targeted Gene Therapies: Tools, Applications, Optimization." Critical Reviews In Biochemistry and Molecular Biology(2012): 264-81. Web of Science. Web. 18 July 2015. Khalili, Kamel, Rafal Kaminski, Jennifer Gordon, Laura Cosentino, and Wenhui Hu. "Genome Editing Strategies: Potential Tools for Eradicating HIV-1/AIDS." Journal of NeuroVirology J. Neurovirol. 21.3 (2015): 310-21. Web of Science. Web. 18 July 2015. Mackay, Joel P., and David J. Segal. Engineered Zinc Finger Proteins: Methods and Protocols. Totowa, NJ: Humana, 2010. Print. Pellagatti, Andrea, Hamid Dolatshad, Simona Valletta, and Jacqueline Boultwood. "Application of CRISPR/Cas9 Genome Editing to the Study and Treatment of Disease." Archives of Toxicology Arch Toxicol 89.7 (2015): 1023-034. Web of Science. Web. 17 July 2015. Yamamoto, Takashi, ed. Targeted Genome Editing Using Site-specific Nucleases: ZFNs, TALENs, and the CRISPR/Cas9 System. Tokyo: Springer, 2015. Print.