2006 10 23 HPLC (for students)
advertisement

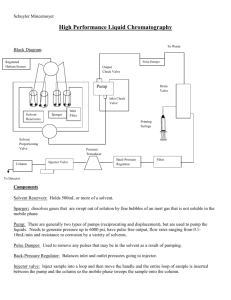
Chapter 25 High-Performance Liquid Chromatography (HPLC) p. 608-634 Harris (6th) p. 556 Harris (7th) Advantages of HPLC two interaction phases stationary and mobile not limited by molecular weight or volatility of analytes room-temperature operation can accommodate thermally unstable samples Limitation of HPLC separation efficiency has a practical upper limit of 100,000 plates/m Experiment 6 Monitoring of Carbonyls by 2,4-Dinitrophenylhydrazine Derivatization and Reversed-Phase High Performance Liquid Chromatography HPLC Solvents 21 solvents of uncompromised quality are available to meet the most demanding applications high assay clean UV trace with a smooth curve throughout low UV absorbance at critical wavelengths UV cut-offs at or approaching the theoretical limit low residue after evaporation Water $63 for a 4 L bottle Solvents (or Mobile Phase) degassed by evacuation, boiling, or purging with He (very insoluble). otherwise, gas bubbles are formed by pressure changes or the mixing of certain solvents Guard column short, expendable (periodically discarded). contains same stationary phase as analytical column. removes impurities in the solvent and sample Sample loop a steel tube with a narrow bore. in various fixed sample volumes (2 μL - 1 mL). LOAD position a syringe is used to wash and load the loop with sample solution at atmospheric pressure. high-pressure flow from the pump to the column passes through the valve Valve handle is rotated 60o INJECT position the contents of the sample loop are injected into the column at high pressure. p. 566 Harris (7th) Selecting the Separation Mode Normal-phase HPLC is the method of choice for analysis of organic solutions (in chloroform). p. 561 Harris (7th) Reverse-phase HPLC for analysis of aqueous solutions (in water) non-polar bonded stationary phase R = -(CH2)17CH3 octadecyl ODS most hydrophobic R = -(CH2)7CH3 octyl intermediate hydrophobicity R = -(CH2)3CH3 butyl R = -(CH2)3C6H5 phenyl polar solvent as mobile phase eluent strength of mobile phase is increased by addition of a less polar solvent Reverse-phase HPLC of non-polar solutes in water Solvents in order of increasing polarity Solvent strength (Eluting power) Hexane, heptane Petroleum ether Cyclohexane . . . . . Ethyl acetate Acetone Acetonitrile n-Propanol Ethanol Methanol Acetic acid Water Highest solubility, shortest tR Non-polar, high eluent strength Polar, low eluent strength Lowest solubility, longest tR pH effect on retention time (and elution order) Octanoic acid and 1-aminooctane were passed through a reversephase HPLC column, using an eluent of 20% methanol in water adjusted to pH 3.0 with HCl. Which compound is expected to be eluted first, and why? Octanoic acid CH3CH2CH2CH2CH2CH2CH2CO2H 1-Aminooctane CH3CH2CH2CH2CH2CH2CH2CH2NH2 p. 565 Harris (7th) Isocratic and Gradient Elution Isocratic elution using a single solvent (or constant solvent mixture composition) maintains the column in equilibrium no re-equilibration time between analyses sample throughput is maximised preferred for routine analysis Gradient elution using two or more solvents of different eluent strengths (or polarities) the volume ratio is varied in a programmed way mixed in solvent chamber before introduction to column enhances resolution of early peaks shortens elution times of late peaks p. 580-583 Harris (7th) Pharmaceutical Analysis by HPLC To look for an impurity in a pharmaceutical formulation/ preparation by analyzing the major component is inherently an unsound approach EXPLOSIVE ANALYSIS by HPLC on Ultracarb 5μ ODS Canadian Explosives Research Laboratory http://www.nrcan.gc.ca/mms/cerl/home_e.htm Qualitative GC HPLC Analysis for peak identification p. 541 Harris (7th) Comparison of retention time with that of an authentic sample of suspected compound. Co-chromatography authentic sample of suspected compound is added to the unknown if the added compound is identical to one component of the unknown, the area of that one peak will increase or with tabulated Kovats retention indexes. For isocratic elution on a single column at different flow rates Capacity factor k = ( tr - tm ) / tm Retention volume Vr = tr F where F = flow rate of mobile phase ( mL/min ) Vr = Vm + K Vs or K = (Vr - Vm) / Vs where K = partition coefficient Vr = retention volume Vm = volume of mobile phase and Vs = volume of stationary phase = volume of solvent inside the gel particles (in MEC) 23-4 Efficiency of Separation p. 511 Harris (7th) Resolution a measure of how well two compound peaks are separated from each other by chromatography R = tr / wav where tr is the difference in retention times between the two peaks and wav is their average base width.