Excess & Limiting Reagents: Chemistry Worksheet
advertisement
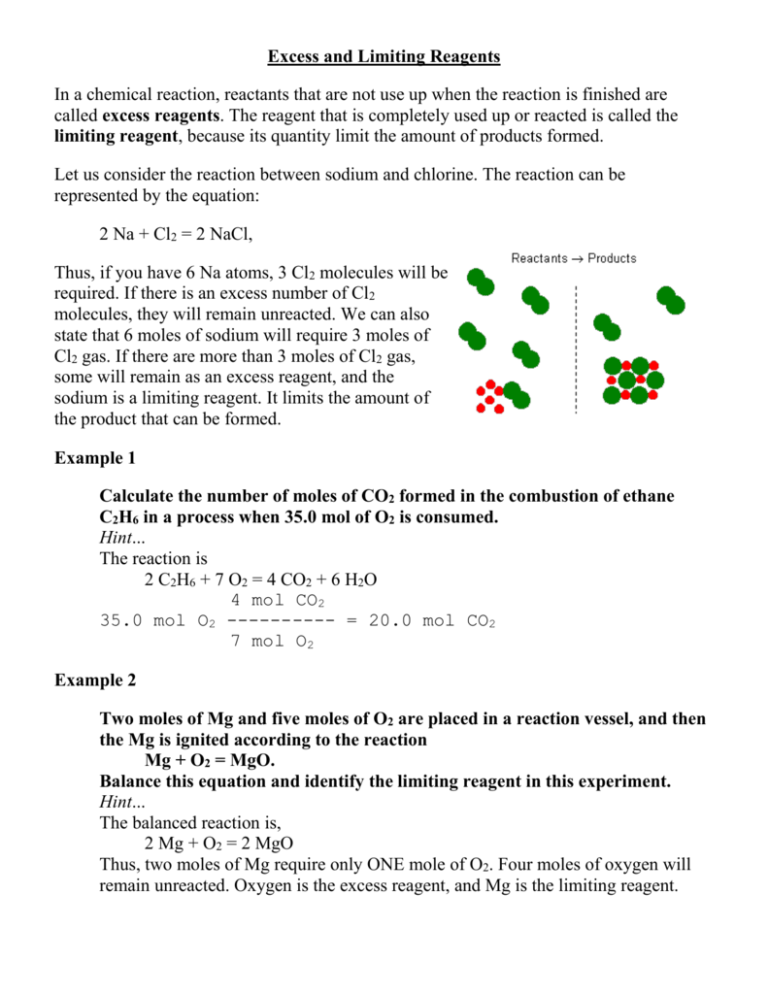
Excess and Limiting Reagents In a chemical reaction, reactants that are not use up when the reaction is finished are called excess reagents. The reagent that is completely used up or reacted is called the limiting reagent, because its quantity limit the amount of products formed. Let us consider the reaction between sodium and chlorine. The reaction can be represented by the equation: 2 Na + Cl2 = 2 NaCl, Thus, if you have 6 Na atoms, 3 Cl2 molecules will be required. If there is an excess number of Cl2 molecules, they will remain unreacted. We can also state that 6 moles of sodium will require 3 moles of Cl2 gas. If there are more than 3 moles of Cl2 gas, some will remain as an excess reagent, and the sodium is a limiting reagent. It limits the amount of the product that can be formed. Example 1 Calculate the number of moles of CO2 formed in the combustion of ethane C2H6 in a process when 35.0 mol of O2 is consumed. Hint... The reaction is 2 C2H6 + 7 O2 = 4 CO2 + 6 H2O 4 mol CO2 35.0 mol O2 ---------- = 20.0 mol CO2 7 mol O2 Example 2 Two moles of Mg and five moles of O2 are placed in a reaction vessel, and then the Mg is ignited according to the reaction Mg + O2 = MgO. Balance this equation and identify the limiting reagent in this experiment. Hint... The balanced reaction is, 2 Mg + O2 = 2 MgO Thus, two moles of Mg require only ONE mole of O2. Four moles of oxygen will remain unreacted. Oxygen is the excess reagent, and Mg is the limiting reagent. Percentage Yield Using stoichiometry, we can mathematically determine the amount of a product that should be formed during an experiment, yet we sometimes find that we don't end up with exactly the right amount of product. Percentage yield allows us to calculate what percent of the expected product we are able to account for by the end of our experiment. The formula that we use is; actual amount of product percentage yield = ------------------------------------------- x 100 expected amount of product Example 1. A student conducts a single displacement reaction that produces 2.755 grams of copper. Mathematically he determines that 3.150 grams of copper should have been produced. Calculate the student's percentage yield. Solve: actual amount of product: 2.755 g expected amount of product: 3.150 g actual amount of product percentage yield = ------------------------------------------- x 100 expected amount of product 2.755g percentage yield = --------------- x 100 3.150g percentage yield = 87.4603174 % percentage yield = 87.46 % Example 2. A student completely reacts 5.00g of magnesium with an excess of oxygen to produce magnesium oxide. Analysis reveals 8.10 g of magnesium oxide. What is the student's percentage yield? First, write a balanced chemical equation for the reaction: 2Mg(s) + O2(g) ----> 2MgO(s) Now, label the given and the unknown and cross out the rest: given unknown 2Mg(s) + O2(g) ----> 2MgO(s) 5.00 g Xg Change the mass given to moles by dividing by the molar mass of Mg. mass given: 5.00g molar mass of Mg: 24.3 g 5.00g Number of moles of Mg = ----------24.3 g/mole Number of moles of Mg = 0.206 moles Compare the molar ratio between the given and the unknown to determine the number of moles produced. Coefficient of Mg; 2 Coefficient of MgO: 2 Number of moles of Mg: = 0.206 moles Number of moles of MgO: = ? number of moles of given number of moles of unknown -------------------------------- = -------------------------------------coefficient of given coefficient of unknown 0.206 moles X moles -------------- = ------------2 2 Number of moles of MgO produced = 0.206 moles Now, change the number of moles of MgO produced to mass by multiplying by the molar mass of MgO. # of moles of MgO = 0.206 moles Molar mass of MgO = 40.3g/mole mass = # of moles x molar mass mass of MgO = 0.206 moles x 40.3 g/mole mass of MgO = 8.30 g Now, you are ready to solve the percentage yield problem. actual mass of MgO produced = 8.10 g expected mass of MgO = 8.30 g actual amount of product percentage yield = ------------------------------------------- x 100 expected amount of product 8.10 g percentage yield = ------------ x 100 8.30 g percentage yield = 97.6 % Percentage purity of a compound In the reaction between aluminium metal and an excess of copper(II) sulphate solution, 4.0 g of aluminium is reacted and 0.48 g of copper metal is formed. Assuming that all the aluminium present reacted to give copper, what is the purity of the aluminium? The equation 2Al(s) + 3CuSO4(aq) 3Cu(s) + Al2(SO4)3(aq) the amount of copper made = 4.8 g/ 64 g mol-1 = 0.075 mol From the equation, the maximum amount of aluminium metal that could be present = 2/3 × the amount of copper made, as the ratio of Al : Cu is 2 : 3. the maximum possible amount of 2 = /3 × 0.075 mol aluminium metal =0.05 mol the maximum possible mass of aluminium = 0.05 mol × 27 g mol-1 metal present =1.35 g =(mass present / total mass of the percentage purity of aluminium used compound)×100 = (1.35 g / 4.0 g)× 100 = 33.75 %






