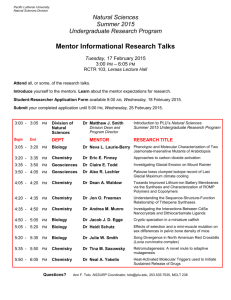
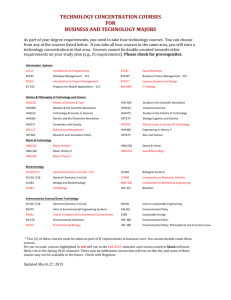
Section 1: Program Conceptual Framework
advertisement

Professional Education Unit University of Kentucky Science Education, Grades 8-12 Biology Education Chemistry Education Earth Science Education Physics Education Initial Preparation Program Submission Date: Fall 2006 UK PROGRAM DESCRIPTION: http://www.uky.edu/Education/EDC/mic/welcome.html GOVERNING KENTUCKY REGULATION: (16 KAR 2:010. Kentucky teaching certificates) http://www.lrc.state.ky.us/kar/016/002/010.htm UNIT POLICY ON ADMISSION, RETENTION AND COMPLETION: http://www.uky.edu/Education/NCATE/coeretention.pdf Contents Section 1: Program Conceptual Framework A. B. C. D. E. F. Conceptual Framework for the Professional Education Unit at the University of Kentucky Mission Statement for the Department of Curriculum and Instruction Mission of the Science Education Program Knowledge Bases for the Science Education Program Performance Standards in the Science Education Program Integration of the Curriculum and Assessment System Section 2: Program Continuous Assessment Plan A. B. C. D. E. F. G. H. I. J. Integration of Program Continuous Assessment Plan with the Unit Assessment System Integration of Program Continuous Assessment Plan with Program Conceptual Framework Program Continuous Assessment Plan Integration with Standards and Program Activities Continuous Assessment Monitoring Checkpoints Multiple Assessments Dispositions and Modes of Assessment Plans for Collecting 8-12 Student Impact Data Candidate and Program Feedback Chart Use of Technology to Support the Assessment System Process to Ensure Accuracy, Fairness, and Consistency of Assessments Section 3: Program Experiences A. B. C. D. E. F. G. H. Overview of Masters with Initial Certification (MIC) Program in Secondary Education Overview of Alternative Route Option in Science Education Delivery of Science Education Program Course Descriptions Integration of Performance Standards into Science Education Program Integration of Code of Ethics into Science Education Program Integration of KERA Initiatives into Science Education Program Integration of EPSP Themes into Science Education Program Section 4: Program Faculty Section 5: Curriculum Contracts 1 Section 1: Program Conceptual Framework The Science Education Program operates under the umbrella of the Master of Arts in Education including initial certification. Science education candidates may pursue program completion in Biology Education, Chemistry Education, Earth Science Education and Physics Education, depending on their subject matter background and personal interests. Although all science education candidates complete similar science education activities and complete the same subject pedagogy courses, they focus on the national and state standards associated with their particular science discipline(s). A. Conceptual Framework for the Professional Education Unit at the University of Kentucky The conceptual framework for the professional education unit at the University of Kentucky (UK) is guided by the theme, Research and Reflection for Learning and Leading. This theme is aligned closely with both the institutional vision and mission of UK and the vision and mission of the professional education unit. The theme reflects and guides how we approach preparation of professional educators within the context of a research extensive, land grant university. Research is a valued activity and tool within UK’s educator preparation programs. Faculty and candidates generate scientific research using a wide range of research methodologies and contribute to the professional literature. Programs use practitioner inquiry and data-based instructional models in applied settings to enhance student learning and professional development. Research findings from the entire field of education inform design of courses, selection of interventions, and features of professional education programs. Reflection is a long-standing aspect of UK’s educator preparation programs and is, in our view, a hallmark of professional practice. Reflective assessment of performance, outcomes, and approaches to problems is a dynamic process appropriate for faculty, experienced educators, and candidates in initial stages of their careers. Candidates are expected to complete numerous reflective activities as they work to meet standards; the goal is to prepare educators who are capable of analysis and problem-solving that will result in improving educational practices and outcomes. Learning is included as a component within our conceptual framework to underscore our commitment to the many facets of learning and to highlight the ways in which our programs conceptualize, promote, and accomplish learning. As a unit, we do not share a single theoretical view of learning. Faculty and candidates conceptualize learning using a wide range of perspectives including behavioral, constructivist, and social. We believe that our diversity of thought enriches and strengthens our unit. The reference to learning in our conceptual framework encompasses learning among all those who participate in our educator preparation programs and those who are affected by the educational efforts of our faculty and candidates. Leading is an expectation that faculty hold for ourselves and an outcome that we promote among our candidates. As members of the educational community at Kentucky’s flagship university, we believe it is our obligation and privilege to provide leadership in educational policies and practices across levels and dimensions of universities, schools, and agencies. We believe that as leaders and followers work together to improve student learning among diverse student populations, we can obtain positive results that improve education in Kentucky and beyond. The four elements of our conceptual framework are synergistic and mutually supportive of our work. Taken as a whole, research, reflection, learning, and leading provide a strong conceptual basis and functional framework for the preparation of educators at the University of Kentucky. B. Mission Statement for the Department of Curriculum and Instruction The mission of the Department of Curriculum and Instruction is to 1) design, develop, and implement programs that will improve the quality of elementary, middle, and secondary education and provide educational leaders; 2) prepare teachers and provide continuing professional development; 3) conduct and disseminate research; and 4) provide services in a variety of educational and professional settings. 2 C. Mission of the Science Education Program The Science Education Program is designed to give candidates the theoretical background and practical skills needed to become effective science teachers. Candidates are introduced to a wide range of instructional materials and ideas that provide opportunities to make decisions relative to appropriate “hands on” and investigative activities for high school students. Candidates are encouraged to be creative and reflective in developing, implementing, and evaluating plans for teaching secondary school science concepts and skills. A strong emphasis is placed upon teaching the processes of science as well as the content of science. Candidates are expected to have high expectations of all students and provide effective instruction for diverse groups of students. Candidates are encouraged to prepare themselves for leadership roles in the schools in which they will serve. D. Knowledge Bases for the Science Education Program The Masters with Initial Certification (MIC) Program and the Science Education Program draw heavily upon what is known about effective teaching and schools. Readings and discussions include a wide range of resources including, but not limited to, philosophy and history of education, working with students from diverse backgrounds, effective teaching strategies, achievement gap, ethics of teaching, intelligence and technology, multicultural issues, asking and answering action research questions, development, learning, motivation, and goals and strategies of teaching high school science. Supporting research and literature for candidates in the Science Education Program include the following: Cohort Classes: Dewey, John. (1938, 1998). Experience and Education. 60th Anniversary Ed. West Lafayette, Indiana: Kappa Delta Pi. Gaines, Ernest J. (1993). A Lesson Before Dying. New York: Vintage. Hersch, Patricia. (1999). A Tribe Apart: A Journey into the Heart of American Adolescence. NY: Ballantine. Ornstein, Allan C., Lasley II, Thomas J. & Mindes, Gale. (2006). Secondary and Middle School Methods. Boston: Pearson, Allyn & Bacon. Soder, Roger, Ed. (1996). Democracy, Education, and the Schools. San Francisco: Jossey-Bass. Strike, Kenneth & Soltis, Jonas F. (2004). The Ethics of Teaching. 4th Ed. New York: Teachers College Press. Technology Education: Sternberg, Robert J. & Preiss, David D., Eds. (2005). Intelligence and Technology. The Impact of Tools on the Nature and Development of Human Abilities. Mahwah, New Jersey: Lawrence Erlbaum Associates. Multicultural Education/Campbellsville Project: Gollnick, Donna M. & Chinn, Philip C. (2006). Multicultural Education in a Pluralistic Society. 7th Ed. Upper Saddle River, New Jersey: Pearson, Merrill Prentice Hall. Core Seminar, Spring: Falk, Beverly & Blumenreich, Megan. (2005). The Power of Questions. A Guide to Teacher and Student Research. Portsmouth, NH: Heinemann. Social Foundations: PBS Series, School: The Story of American Public Education (2001). The attendant reader of the same title by Sarah Mondale (Beacon Press, 2001), is also recommended. 3 Special Education: Blackboard: Access via https://myuk.uky.edu or http://elearning.uky.edu Rose, David H. and Meyer Anne (2002) Teaching Every Student in the Digital Age: Universal Design for Learning. ASCD: ISBN: 0-87120-599-8. Educational Psychology (Development, Motivation, and Learning): Mayer, R.E. (2001). What good is educational psychology? The case of cognition and instruction. Educational Psychologist, 36(2), 83-88. Behavioral Learning Theory Web Quest http://suedstudent.syr.edu/~ebarrett/ide621/behavior.htm Cognitive Theory Web Quest http://suedstudent.syr.edu/~ebarrett/ide621/cognitive.htm Social Learning Theory Web Quest http://suedstudent.syr.edu/~ebarrett/ide621/social.htm Huitt, W. (1997). Motivation to learn: An overview. Educational Psychology Interactive. Valdosta, GA: Valdosta State University. http://chiron.valdosta.edu/whuitt/col/ Maehr, M.L. & Midgley, C. Enhancing student motivation: A Schoolwide approach. Educational Psychologist 26, 3 & 4 (1991): 399-427. Furrer, C. & Skinner, E. (2003). Sense of relatedness as a factor in children’s academic engagement and performance. Journal of Educational Psychology, 95(1), 148-162. Learner-Centered Psychological Principles www.apa.org/ed/lcp2/lcp14.html Brett, A., Smith, M., Price, E., & Huitt, W. (2003). The affective domain. Educational Psychology Interactive. Valdosta, GA: Valdosta State University. Retrieved from http://chiron.valdosta.edu/whuitt/col/affsys/affsys.html. Huitt, W. (1997). Socioemotional development. Educational Psychology Interactive. Valdosta, GA: Valdosta State University. Retrieved from http://chiron.valdosta.edu/whuitt/col/affsys/erikson.html Secondary Science Methods: National Research Council (1996). National science education standards. Washington, DC: National Academy Press. (http://www.nap.edu/readingroom/books/nses/html/) Kentucky High School Core Science for Assessment for both Middle School and High School Science http://www.education.ky.gov/users/jwyatt/CCA%204%201%20FINAL/CCA_41.doc Kentucky Program of Studies (http://www.education.ky.gov/users/jwyatt/POS/POS.pdf) Kentucky New Teacher Standards http://www.kyepsb.net/teacherprep/newteacherstandards.asp#std.1 . American Association for the Advancement of Science. (1989). Science for all Americans: A project 2061 report on literacy goals in science, mathematics, and technology. Washington, DC: American Association for the Advancement of Science. American Association for the Advancement of Science. (1993). Benchmarks for science literacy. New York: Oxford University Press Appel, Kenneth, John Gastineua, Clarence Bakken, David Vernier. (2003). Physics with Computers. Beaverton, Oregon: Vernier Software & Technology. 4 Billmeyer, Rachel., Mary Lee Barton. (1998). Teaching reading in the content areas: If not me, then who?. 2nd Edition. Mid-Continent Regional Educational Laboratory. Driver, Rosalind., Ann Squires, Peter Rushworth, Valerie Wood-Robinson. (1994). Making Sense of secondary science: Research into children’s ideas. : reprinted 2005. New York: Routledge Falmer Hazen, Robert M., James Trefil. (1991) Science Matters: Achieving scientific literacy. New York: Anchor Books. Holmquist, Dan D. and Donald L. Volz. (2003). Chemistry with computers. 3rd Edition. Beaverton, Oregon: Vernier Software & Technology. Johnson, Robyn L., Gretchen Stahmer DeMoss, Richard Sorensen. (2003) Earth science with computers. Beaverton, Oregon: Vernier Software & Technology. Jones, L.R., I. Mullis, S.A. Raizen, I.R. Weiss, and E.A. Weston. (1992). The 1990 science report card: NAEP’s assessment of fourth, eight, and twelfth graders. Washington, DC: U.S. Department of Education. Keeley, Page. (2005) Science Curriculum Topic Study: Bridging the gap between standards and practice. Thousand Oaks, California: Corwin Press Kober, Nancy. (1992). What we know about science teaching and learning. Washington, DC: Council for Educational Development and Research. Masterman, David and Scott Holman. (1997) Biology with Computers Using Logger Pro. Beaverton, Oregon: Vernier Software Morholt, E. and Paul Brandwein. (1989). A sourcebook for the biological sciences (third edition). San Diego: Harcourt Brace Jovanovich, Inc. National Academy of Sciences. (1990). Fulfilling the promise: biology education in the nation’s schools. Washington, DC: National Academy Press. National Academy of Sciences. (1998). Teaching about evolution and the nature of science. Washington, DC: National Academy Press. National Research Council (2005). How students learn science in the classroom. Washington, DC: National Academy Press. National Research Council (1996). National science education standards. Washington, DC: National Academy Press. National Science Teachers Association. (1996). NSTA Pathways to the science standards: Guidelines for moving the vision into practice. Arlington, Virginia: National Science Teachers Association. Volz, Donald L. and Sandy Sapatka (2003). Physical science with computers. 3rd Edition. Beaverton, Oregon: Vernier Software. E. Performance Standards in the Science Education Program The Science Education Program aligns itself with and expects candidates to meet the following performance standards: Kentucky New Teacher Standards, the Unit Functional Skills and Dispositions, the Unit Technology Standards, and the National Science Teachers Association/National Council for Accreditation of Teacher Education Secondary Science Education Standards. 5 F. Integration of the Curriculum and Assessment System The Science Education Program for initial preparation and certification enables candidates to meet required standards for new teacher practice, leadership, research, and reflective practice through a variety of designed program experiences. The program allows candidates to integrate a number of ideas related to content and professional knowledge. With the exception of six hours of graduate content work, content preparation occurs prior to admission to the teacher preparation program. The program provides a variety of course work leading to a functional knowledge of student learning, development, and motivation relative to teaching the content area. A large block of field experiences coupled with classroom work enables candidates to meet program goals and standards through performance. Candidate performance is evaluated through the continuous assessment system and numerous products produced during their work. Assessments include interviews, project feedback, and an extensive use of the portfolio. Information gleaned from candidate work is used to make adjustments in activities and experiences provided for candidates. In addition, there is a close working relationship between university faculty and colleagues in the field. Practicing teachers are encouraged to provide feedback regarding strengths and weaknesses of the program and ways in which the preparation of teachers might be improved. Candidates being observed by university supervisors provide systematic feedback regarding needs for modifying and improving the teacher preparation program. 6 Section 2: Program Continuous Assessment Plan A. Integration of Program Continuous Assessment with the Unit Assessment System The Science Education Program utilizes a continuous assessment system designed to assess candidate proficiency and program effectiveness. Continuous assessment of candidates involves a developmental approach to educator preparation in which candidates are expected to progress toward mastery of standards as they practice and gain competence with increasingly complex pedagogical and professional tasks. Through continuous assessment, the program monitors candidate performance over the duration of the program. Candidates are expected to progress over time as their involvement with program activities increases. Candidates are expected to meet or exceed minimum program expectations upon entry into the program. As involvement in classes and field experiences increase, a higher level of performance is expected. The Science Education Program Faculty uses candidate data to measure the progress of individual candidates throughout the program and then uses aggregated candidate data in the process of determining the effectiveness of the program. Selected data items collected on candidate proficiency and program effectiveness in the Science Education Program is also fed into the unit assessment system. For example, all programs collect candidate data using the Continuous Assessment Review (CAR). The CAR is used at program admission, retention, and exit transition points to record candidate performance on the appropriate Kentucky-adopted educator proficiency standards, the Unit Functional Skills and Dispositions, and the Unit Technology Standards. The analysis of candidates’ performance on the National Science Teachers Association (NSTA) standards also informs the unit assessment system. In addition, data from the New Teacher Survey administered by the Kentucky Education Professional Standards Board are reviewed by the program faculty. These data are an essential element of the program evaluation component of the unit assessment system. Finally, a wide range of basic data items, i.e., grade point averages (GPAs), admissions test scores, student teaching evaluations, and PRAXIS II examination pass rates, is reviewed by the Science Education Program Faculty, audited and monitored at the unit level, and fed into the unit’s comprehensive data system. These data sets constitute important information for program development and unit operations. B. Integration of Program Continuous Assessment Plan with Program Conceptual Framework Program and end-of program assessments are evaluated in the context of candidates having the theoretical background and practical skills needed to become effective science teachers. Candidates are expected to make informed decisions in the selection of developmentally appropriate, hands-on, investigative, process oriented, activities for high school students. Candidates are expected to be creative and reflective in developing, implementing, and evaluating plans for teaching secondary school science concepts and skills. Candidate dispositions must demonstrate sensitivity to diverse student populations, the need for multiple teaching and assessment strategies, a commitment to asking and answering teaching questions using appropriate literature and inquiry strategies, a preference for reflective teaching activities, and a desire to serve as a change agent (leadership role) within the school and school system. C. Program Continuous Assessment Plan Integration with Standards and Program Activities A primary focus of the Science Education Program is to meet multiple standards proposed by state and national agencies (see Section 3). Collective and collaborating data are used to recommend program changes/improvements. D. Continuous Assessment Monitoring Checkpoints Formal assessments are conducted at the following points: Checkpoint 1: Admissions to the MIC Program (Prior to Fall Semester) Checkpoint 2: Retention (End of Fall Semester) Checkpoint 3: Exit (End of Spring Semester) Follow-up: KTIP and Follow-up Surveys E. Multiple Assessments Multiple assessments are used to determine candidate progress throughout the program. These assessments inform the program of candidate progress and provide input regarding program adjustments. 7 Early Assessments: Quality of References Presented For Admission to Program Successful Completion of Required Courses – 128 Semester Hours and Undergraduate Degree Course Content Grades – 33 Semester Hours in the Science Major with a Minimum GPA of 2.50 Hours of Support Course Work – 24 Hours+ in Support Area with Minimum GPA of 2.50 Overall Course Grades – 128 Semester Hours with GPA of 2.75 (Graduate School Requirement) GRE Scores (Verbal, Quantitative, Writing) – 400, 400, 4 (ACT 18, PRAXIS I, or Composition Course with a Minimum Grade of B in Lieu of 4 Writing Score) Advising Sessions – Monitor Course Progress and Adherence to Program Requirements Communication Skills (Oral and Written) – Writing Sample and Ability to Communicate with Program Admission Interviewers Understanding of Requirements for Becoming a Teacher – Program Admission Interview Dispositions Exhibited for Becoming a Successful Science Teacher – Program Admission Interview and Dispositions, Technology, and New Teacher Standards Program Assessments: University Classroom Work (Projects, Papers, Presentations) Classroom Examinations Advising Sessions – Monitor Progress in Program and Problems Encountered by Candidate Field Placements – Monitor Performances Exhibited in Field Experience Mid-Program Assessments – Dispositions, Technology, and New Teacher Standards Planning Skills – Evaluation of Lesson and Unit Plans Prepared in Classes Observations in Student Teaching –Informed Selection and Implementation of Appropriate, Planning, Teaching Strategies, Student Assessment, Ability to Analyze and Discuss Teaching Effectiveness Selection and Development of Portfolio Artifacts (Mid-point Review) End-of-Program Assessments: Written Masters Degree Examination Portfolio (Portfolio Artifacts Are Uploaded to An Online, Electronic Portfolio System) Portfolio/Exit Conference Written Program Surveys – Student and Cooperating Teacher Success in Finding Employment as Practicing Teacher Continuous Assessment – Dispositions, Technology, and New Teacher Standards PRAXIS II Scores in Content Area Follow-up Assessments: Performance in KTIP Experience Written Program Surveys – Intern Teacher and Resource Teacher Continuation of First Year Teacher Contract F. Dispositions and Modes of Assessment The combined program faculties of the UK educator preparation unit have established five (5) skills and dispositions that underlie all UK educator preparation programs. These skills and dispositions have been adopted and endorsed by the Science Education Program Faculty. The required skills and dispositions are as follows: Functional Skill and Disposition 1: Candidates communicate appropriately and effectively. Communicates successfully in formal presentations Communicates successfully in small groups and/or informal settings Uses nonverbal communications skills successfully Communicates successfully in writing (reports, essays, letters, memos, emails, etc.) Functional Skill and Disposition 2: Candidates demonstrate constructive attitudes. Demonstrates knowledge and command of sociocultural variables in education Demonstrates constructive attitudes toward children, youth, parents, and the community Demonstrates awareness and acceptance of diversity in educational settings. 8 Functional Skill and Disposition 3: Candidates demonstrate ability to conceptualize key subject matter ideas and relationships. Accurately states key subject matter ideas Explains key subject matter ideas Tailors key subject matter ideas to diverse populations Addresses misconceptions among students about key subject matter ideas Identifies real life examples to enhance student learning of key subject matter ideas Function Skill and Disposition 4: Candidates interact appropriately and effectively with diverse groups of colleagues, administrators, students, and parents in educational settings. Demonstrates acceptable educator behavior in diverse educational settings Demonstrates adaptability in reflecting on self in relation to diverse groups Functional Skill and Disposition 5: Candidates demonstrate a commitment to professional ethics and behavior. Demonstrates understanding of the Kentucky School Personnel Code of Ethics Complies with all legal requirements for educators in a knowledgeable and timely manner Demonstrates understanding of ethical issues related to own professional certification area The Science Education Program Faculty uses the unit Continuous Assessment Review form to rate each candidate at each of the three assessment points on the unit dispositions. When candidates consider applying for a professional preparation program, they are provided with the unit’s set of Functional Skills and Dispositions and a self-assessment form so that they can begin reflecting on their own capability with each of the skills/dispositions. Candidates use artifacts as evidence of their capabilities with each of the skills/dispositions items. Faculty accumulate evidence from coursework, student portfolios, interviews, and supervisor ratings to determine their ratings. Candidates have an opportunity to reflect with the faculty about the ratings and can use faculty feedback to accumulate new opportunities to develop their skills and dispositions and to demonstrate them via artifacts. G. Plans for Collecting 8-12 Student Impact Data The Science Education Program is keenly aware of the importance of the issues of P-12 teacher quality enhancement, promotion of increased achievement for P-12 students, and closing the achievement gaps between P-12 student populations. The program is committed to developing future teachers who will help address these issues. The program develops candidate research capability and studies the extent to which pedagogical activities affect P-12 learning. Coursework requires candidates to plan, implement, and assess lessons and units. Candidates analyze the results of their efforts, with increasingly sophisticated tools as their experience with research methods grows. Candidates also gain experience and facility in utilizing the results of CATS assessments to understand student needs and to interpret student performance behaviors. In addition, the program plans to work with graduates of the program to collect aggregated summary performance data from the Commonwealth Accountability Testing System (CATS), particularly subject-specific summary data. The Science Education Program has plans to collect student achievement data from the CATS for teachers successfully completing the MIC Program. This would occur during teachers’ first-year participation in KTIP. Given the variety of science content areas, student variables, and teaching/learning environments, this could prove to be an important, but formidable, task. 9 H. Candidate and Program Feedback Chart Green = Candidate Feedback Red = Program Feedback Admission into program Exit portfolio feedback; Exit interview feedback; Student Teaching Observation Evaluations Checkpoint 3 Exit Assessment Data Initial Student Data; Unit pass rates on PRAXIS II Checkpoint 1 Admissions Data Successful completion of the Program; Job Placement Rates; Student Evaluations; Mentor Teacher Evaluations Successful completion of Methods Course; Effective Evaluations of Retention Portfolios; Faculty teaching performance Checkpoint 2 Mid-Point Retention Assessment Data Retention portfolio feedback 10 I. Use of Technology to Support the Assessment System Candidates admitted to the program begin developing portfolio materials during each semester of the program under the guidance of the Program Chair and other course instructors. Portfolio materials are uploaded to an online portfolio system. Candidates post artifacts and demonstrate ways in which they are meeting each New Teacher Standard. These materials are reviewed online by the science education faculty person at the mid-point of the program and again at the end of the program. In addition to portfolio materials uploaded by candidates, student teaching observation evaluations can be posted to the candidates’ online portfolios. At the unit level, the results of candidate reviews by the Science Program Faculty, in the form of admission to program and completion from program, are recorded in the unit database and information system. These data are readily retrievable and used in studies of cohort characteristics. Data relating to testing, GPA, and progress through programs are also recorded and available. The unit web portal system, which is under development, will permit the direct entry of candidate performance assessment data into the comprehensive database system. One of the first of the portal-based data entry modules will allow program faculties to directly enter continuous assessment ratings (CAR) into candidate records. While the necessary portal modules have been under development, the Science Education Program Chair has entered the CAR ratings into pre-formatted Excel Spreadsheets for submission and storage at the unit level. J. Process to Ensure Accuracy, Fairness, and Consistency of Assessments The Science Education Program, along with the Mathematics Education Program, is managed by the Math and Science Program Faculty. This group consists of science educators, mathematics educators, science faculty, mathematics faculty, graduate mathematics and science education candidates, and practicing science and mathematics teachers. The group represents a broad constituency, and it is intended that this structure provide admission and retention processes that are accurate, fair, and consistent. For example, candidates for program admission are reviewed by two- and three-member interview committees representing membership of the Program Faculty. Following the interview, interviewers share applicants’ admission portfolios, along with rating scales and notes, with the entire membership of the Program Faculty. It is believed that this process provides a mechanism that is accurate, fair, and consistent for all candidates for admission and all other decisions involved in the management of the program. 11 Section 3: Program Experiences Initial teacher certification in science education is available through two routes in the Science Education Program at the University of Kentucky. The first is through the Masters with Initial Certification (MIC) Program, in which candidates enter the program with an undergraduate major in the sciences, or the equivalent, and complete a master’s degree that leads to initial certification at the Rank II level in Kentucky. The second is an alternative route option that is available to teachers who are initially employed on a temporary certificate by partnering school districts. These two options are described in the following sections. A. Overview of the Masters with Initial Certification (MIC) Program in Secondary Education The Master of Arts in Secondary Education with Initial Certification (MIC) at the University of Kentucky (UK) is a unique and intensive program of 34 credit hours leading to both a master’s degree and initial teacher certification in Kentucky. The format of the program requires that candidates participate in challenging graduate coursework and extensive field preparation that promotes an understanding and synthesis of theory and practice. The MIC offers five content-area programs, including business/marketing, English, mathematics, science, and social studies. As a doctoral-granting university with the designation of RU/VH: Research University (very high research activity), as designated by Carnegie Classifications, UK promotes an intense commitment to the generation of research by faculty and candidates and also promotes the use of research-based practices. This commitment to research is reflected in the institutional and unit mission and vision statements. The professional education unit at UK has adopted the model of preparing educational professionals for Research and Reflection for Learning and Leading, befitting the university’s role as an RU/VH. The MIC, as a master’s level program, is committed to this model. Our candidates learn to appreciate, generate, and adapt research in a reflective manner to promote student learning and to enhance their abilities as teacher leaders. Each candidate in the MIC participates in two cohort groups, the common core cohort and the content-area cohort. The common core cohort is the primary organizing unit of the fall semester. The common core cohort is built around a high degree of cooperation between the professional education unit and local high schools. Each candidate is assigned to a common core cohort made up of candidates from each of the content areas. These cohorts are lead by faculty in the Department of Curriculum and Instruction (C&I) and meet in four area high schools in three counties. The common core cohort classes have two components. At the beginning of the semester, candidates meet with their cohort leaders at their assigned high schools to lay the groundwork for their field placements. In the cohort classes, candidates are introduced to general pedagogical issues, including the relationship of educational philosophy to practice, classroom management techniques, theories of adolescent development and learning, instructional strategies, and ethical issues associated with being a high school teacher. To supplement this coursework, candidates also work with other C&I faculty to increase their knowledge of multiculturalism and its relationship to teaching and learning and how to incorporate technology in their classrooms. The second component of the common core cohort is a six-week field placement in one of four cohort schools. The placement is four days per week, four hours per day. Candidates are placed in their content-area departments and observe and work with a wide variety of teachers and administrators. They are required to become immersed in the school culture through observations, meetings, teaching, and coursework. They complete major cohort projects on each of the following: interdisciplinary instruction, service learning, classroom management, and tutoring/mentoring. In addition to the common core cohort classes, candidates take classes in educational psychology, educational foundations, and special education. These classes meet on campus before and after the field experience. The premise is to allow candidates an opportunity to become acquainted with educational theories, practices, and special issues in these areas before interacting with students and then to reflect on these elements once the fall field experience is complete. Faculty members from these classes also have candidates complete projects related to their field experience. Finally, each candidate takes a content-area methods course. As with the common core classes, the methods courses meet weekly throughout the semester – before, during, and after the seven-week field placement. Content-area professors focus on content-specific pedagogy and work closely with all other faculty to ensure candidates experience a well-integrated 12 curriculum that ties all coursework, field activities, and assignments together to promote the synthesis of theory and practice. Fall Semester MIC Courses Course Hours Title EDC 730* 3 Foundations of Pedagogical Theory (Common Core Cohort) EDC 777* 3 Practice in the Secondary School (Common Core Cohort) EDS 558 1 Issues in Special Education EDP 658 1 Problems in Educational Psychology EPE 773 1 Seminar in Educational Policy Studies and Evaluation EDC 63x 3 Special Methods in (content area) *EDC 730 and EDC 777 are being replaced with new identifying course numbers – EDC 645 and EDC 646, respectively. The content of the courses will remain the same. The organizing element of the spring semester is the content-area cohort. During the spring semester, each candidate completes 16 weeks of student teaching in an area high school. The focus in the spring is on honing their skills as teachers of a specific content and applying the theoretical knowledge gained during the fall semester. Unlike the fall field experience, the spring student teaching assignment is with an individual teacher in one classroom. After an initiation period of a week or two, candidates begin taking responsibility for planning and teaching the classes and eventually assume a full teaching load under the supervision of the cooperating teacher and a university supervisor. Content-area professors meet regularly throughout the spring semester with student teachers to continue their instruction in content-specific pedagogy. In addition to student teaching, candidates participate in an evening seminar on campus. The primary focus of the spring seminar is on learning classroom inquiry techniques. Classroom inquiry involves such strategies as action research, case study, personal narrative, and ethnography for the purpose of improving teaching and learning. Each candidate develops a classroom inquiry project based on their student teaching experience. At the end of the semester, they are required to give oral and written presentations on their inquiry projects as their culminating project for the spring seminar. Additionally, during the spring seminar, candidates continue their work with faculty on incorporating multiculturalism and technology in their classrooms. Finally, during the spring semester, each candidate takes a course in educational leadership. In this one-hour course, candidates are introduced to many legal and ethical issues they encounter in their classrooms. Once again, the educational leadership faculty work closely with C&I faculty to integrate the content and instruction with that of the other courses the candidates take. Spring Semester MIC Courses Course EDC 746 EDC 777* EDA 770 Hours 9 3 1 Title Subject Area Instruction in the Secondary School (Student Teaching) Teaching Across Curriculum in Secondary Schools Topical Seminar in Educational Leadership: Teacher Leadership for the MIC Program *EDC 777 is being replaced with a new identifying course number – EDC 745. The content of the course will remain the same. Candidates take their elective courses during the summer sessions before and after the cohort experiences of the academic year or in the evenings during the academic year. They must take six hours of electives in their content area and three hours of electives in curriculum and instruction. Advisors work closely with candidates to choose elective classes that fill any gaps in the candidates’ content or pedagogical knowledge. The entire program is dedicated to the development of professional educators who understand theory and practice and how the two elements work together to create positive learning experiences for high school students. To ensure that our candidates are progressing toward that goal, mid-term assessments are made at the end of the fall semester in both the common core cohort and in the content-area cohort. The common core assessment involves writing an in-depth philosophy of education, using the knowledge gained from all courses and field experience during the semester. The content-area instructors use portfolio assessment. In the spring, each candidate is required to take a master’s comprehensive examination and to present the results of their classroom inquiry projects as part of their culminating activities. The final 13 assessment in each content area is based on candidates’ portfolios. They also are required to pass the Praxis II exams in pedagogy and their content areas prior to receiving a recommendation for certification. The MIC at the University of Kentucky is a unique teacher preparation program. We seek candidates with proven ability in various content areas and then provide them with the theoretical and practical knowledge, skills, and dispositions to become effective classroom teachers. For admission into the program, each candidate must: have completed a bachelor’s degree in their content area or an equivalent, have an overall GPA of 2.50 and a content-area GPA of 2.75, have taken the Graduate Record Examination (GRE), and have completed an interview with content-area faculty. 1. 2. 3. 4. Program faculties in each content area are the official governing bodies for the respective programs. As such, they are responsible for admission of candidates to their respective programs; for continuous assessment of candidates throughout their programs; for reviewing candidate data to inform program improvement efforts; and for initiating course and program changes based upon feedback from candidates, graduates, and their supervising/mentor teachers in the schools. B. Overview of the Alternative Route Option in Science Education The alternative certification option in science education at the University of Kentucky is designed for the preparation of secondary science teachers and tailored to meet the needs of individual candidates who qualify for admission to the program. This two-year program enables candidates with concurrent employment or sponsorship in a school district to achieve Rank III certification and potentially rank II certification. The alternative certification option at UK requires candidates to have a signed contract with a local school. Successful teaching is a complex, multi-layered, developmental endeavor that involves a unique combination of knowledge and skills on different dimensions; therefore, a special emphasis is placed on the concept of Teaching as Inquiry in the program. At its most basic level, though, it involves continuous inquiry around key questions related to how students learn. This program focuses on helping candidates become adept at this process of inquiry about teaching and learning. As candidates progress through this program of supervised field experiences, structured seminars, and problem-based activities, they acquire the knowledge and skills needed to become the kind of reflective teacher-scholars needed to help students succeed. The program is organized around several key dimensions of teaching that the Science Education Program Faculty view as essential aspects of teacher expertise. The following dimensions of teacher knowledge guide the curriculum comprised of field experiences and focused seminars: Teaching as an Instructional Activity involves the ability to apply knowledge, transform knowledge into practice, and develop curriculum to support diverse learners in various environments utilizing appropriate classroom management and technology skills. Teaching as a Disciplinary Activity involves candidates acquiring knowledge in the disciplines that are the foundation of the teaching profession and the focus of the subject area candidates will teach. Candidates continue to broaden and deepen their disciplinary expertise over the course of their careers. Teaching as a Socio-Cultural Activity examines the many social, cultural, economic, and political factors in our society that affect what happens in schools and in the way we raise and provide for children. Candidates understand how values, beliefs, language, and religion intersect with their students’ identity and motivation, and how these affect equity and access to learning opportunities for students. Teaching as a Reflective Activity emphasizes that candidates think about what they do and why they do it as a daily challenge in teaching. Candidates will become increasingly purposeful in what they do and be able to consider the consequences of their decisions. Candidates will ask if their choices and actions are good to continue or need to change. This reflective knowledge is achieved through continuous informal and formal inquiry among candidates, university faculty, and school personnel. Teaching as a Developmental Activity suggests candidates understand how children change as they grow. Candidates will be able to guide students’ learning in ways appropriate to their cognitive, physical, and emotional development. Teaching as a Moral/Ethical Activity indicates every action has a moral component. Candidates will recognize the moral component of their work so they can create safe, supportive environments that welcome differences 14 of opinion and diversity of experiences and learning styles. Candidates must model ethical decision-making as it pertains to the classroom and link the classroom to the social and natural environments in which we live. Teaching as a Collaborative Activity emphasizes the value and importance of good working relationships among candidates, faculty, colleagues, students, and family members. Candidates also need to work in partnership with other school support staff, education administrators, community leaders, and social service providers. Admission The alternative certification option in science education is designed for teachers holding temporary certification who wish to remain employed in the area while gaining certification at the same time. The program condenses educational experiences from UK’s ongoing program (i.e., MIC), but does not compromise the intellectual rigor expected of all candidates who enroll in the university. The two-year program culminates in full certification. Individuals who want to teach and who hold at least a bachelor’s degree in science are provided this option of an alternative route to teacher certification. Candidates complete an application for admission to the alternative certification program and for admission to the Graduate School as a post-baccalaureate student. Applicants are reviewed by the Science Education Program Faculty and must meet program expectations of this faculty group. Candidates may choose to work toward certification only or have some course work apply toward an eventual master’s degree. Transfer work from post baccalaureate and other institutions is limited to nine (9) semester hours. Candidates desiring an eventual master’s degree must apply for, and be admitted to, full admission to the Graduate School’s master’s degree program in secondary education. As a prerequisite to admission, all candidates must be employed by a school district in the University of Kentucky service area. This program seeks candidates with a diversity of exceptional life experiences. These individuals might be “career changers” or persons with exceptional experiences who can bring to the classroom strengths and abilities not typically found in traditional candidates. Eligibility As mentioned above, to be eligible for the program, the candidate must be employed or sponsored by a participating school district. The terms of employment or sponsorship must enable the candidate to complete all necessary school-based experiences required in the program. Coursework The alternative certification program in science education is an intensive two-year, part-time program. Candidates are enrolled in the fall and spring semesters and in the summers. The following courses are required during the first and second academic year: Academic Year One (Evenings or Weekends) Fall Semester Secondary Science Methods Course (EDC 634) Field Experiences in Secondary Education (EDC 362) 3 hours 3 hours Spring Semester Introduction to Education (EDC 501, Teaching Internship) Classroom Management/Discipline (EDC 610) 3 hours 3 hours Academic Year Two (Evenings or Weekends) Fall Semester Introduction to Education (EDC 501, Teaching Internship) 3 hours Spring Semester Introduction to Education (EDC 501, Teaching Internship) 3 hours During the first and second summers (and/or the second year of the program), participants are required to complete 6 hours in the following areas: 1) working with students with special needs, and 2) learning, development, and motivation. Participants are required to complete a minimum of three (3) semester hours in each of these two categories. Technology, Teaching, Humanistic, and Multicultural Studies will be integrated into the teaching internship courses. 15 Prior to admission to the alternative certification program in science education, candidates submit a list of all contentrelated courses taken. Advisors review all course work and identify any deficiencies in the major and/or support areas. All deficiencies must be completed prior to being certified. Where appropriate, advisors may substitute graduate level courses for existing subject area requirements. As they are available, content courses may be taken in the evenings of the fall and the spring semesters. Some content courses may be available during summer school. Other content courses can be taken at community colleges, private colleges, or regional universities. The number of content courses required varies from candidate to candidate. The number is a function of an individual’s previous content courses and certification requirements for the Science Education Program. Clinical and Field Experiences Clinical and field experiences are completed in the classrooms to which the candidates are assigned. These experiences are correlated with coursework and seminars in which candidates are concurrently enrolled. The first-year experiences serve to meet practicum and student teaching requirements. Upon completion of the alternative certification program and issuance of the Rank III certification, teachers participate in the KTIP Program. KTIP Program Following the second year of this program, candidates participate in the Kentucky Teacher Internship Program (KTIP) year in which the intern teacher will be observed, assessed, and assisted by the three-member internship committee comprised of the principal, resource teacher, and teacher educator. Assessment As candidates progress throughout the program, they are evaluated continuously through a series of performance assessments that incorporate these dimensions: the Kentucky New Teacher Standards, the NCATE/NSTA Science Education Standards, the Unit Functional Skills and Dispositions, and the Unit Technology Standards. Assessment begins with the admissions process and continues throughout the program. Formal assessments occur at the end of each semester. A formal exit assessment is completed at the end of the two-year period, conducted by the Science Education Program Faculty. These assessments include existing retention and exit assessment rules established for teacher education candidates in the professional education unit. Items to be assessed include portfolios, on-demand writing samples, classroom performance, course materials, student learning outcomes, and candidate standardized test results, including the appropriate PRAXIS II content examination and the Principles of Learning and Teaching Test. C. Delivery of Science Education Program Candidates in the Masters with Initial Certification Program are organized into two cohorts: an interdisciplinary cohort and a disciplinary methods cohort. In the fall semester, candidates in the interdisciplinary cohort are organized into cohorts at four schools including Bryan Station High School, East Jessamine High School, Tates Creek High School, and Woodford County High School. Candidates are assigned to cohorts across disciplines. Instructors meet the cohort classes in the respective schools. The science teaching methods course is currently being taught in a science classroom at Tates Creek High School. Student teaching seminars, organized by content areas, are held in local schools. D. Course Descriptions Masters with Initial Certification (Grades 8-12) Coursework in Science Education: EDC 634 Science Pedagogy in the Secondary School (3 credits) Through campus and school-based experiences, candidates will learn how to engage young people in learning mathematics and how to make decisions about planning instruction and develop assessment based on a sound knowledge base for applying content, materials, and methods (including educational technology) appropriate for high school students. EDC 746 Student Teaching in Science (9 credits) The purpose of student teaching and the student teaching seminar is to help student teachers continue to develop their knowledge, strategies, and the skills necessary in order to become a successful and productive science teacher capable of leading in the profession. With the support of cooperating teachers in area public schools, the course instructor, and university field supervisors, student teachers apply theories, methods, and techniques they have learned in the past in addition to what they will learn during the concurrent student teaching experiences. 16 EDC 777 Practice in the Secondary School (MIC Cohort) (3 credits) Classroom management, technology, and multicultural education are addressed. The purpose of this course is to give both a sound theoretical foundation and in-depth experiences enabling one to become a professional educator who utilizes research and reflection in order to learn and lead in the classroom. EDC 730 Problems of the School Curriculum (3 credits) Problems in the field of the school curriculum and in the preparation of instructional materials. Classroom management, technology, and multicultural education are addressed. The purpose of this course is to give both a sound theoretical foundation and in-depth experiences enabling one to become a professional educator who utilizes research and reflection in order to learn and lead in the classroom. EDP 658 Problems in Educational Psychology (1 credit) Special topics in psychological theories and research applicable to educational practices. May be repeated to a maximum of six credits. Prereq: Consent of instructor. EDS 558 Issues in Special Education (1 credit) In-depth study of a current and topical problem or issue in the education of exceptional children and youth. May be repeated to a maximum of nine credits. A title is assigned each time the course is offered. (Same as RC 558). EPE 773 Seminar in Educational Policy Studies and Evaluation (1 credit) Examination of selected problems in educational policy studies and evaluation. The implicit objective of social foundations courses (e.g., Philosophy of Education; History of Education, Politics of Education, etc.) is to assist candidates in understanding the social nature of education in our society; to think critically and reflectively about education; and to recognize education as an area of inquiry in which systematic study can benefit practice. EDL 770 Topical Seminar in Educational Leadership (1 credit) Advanced graduate students enroll in this topical seminar to enhance their portfolios for educational leadership through concentrated study of innovations in the specialized functions of administration. These specializations include, but are not limited to, the study of curriculum and instructional leadership, educational law, personnel administration, school and community relations, education for diverse populations, budgeting and financing of schools. E. Integration of Performance Standards into Science Education Program The Science Education Program is aligned with state, institutional, and national standards, which include the Kentucky New Teacher Standards, the Unit Functional Skills and Dispositions, the Unit Technology Standards, and the National Science Teachers Association Standards. A description of the alignment of the science education curriculum and experiences with these standards sets follows in this section. Kentucky New Teacher Standards for Preparation and Certification The Kentucky New Teacher Standards serve as a primary focus throughout the program. Using the continuous assessment process, candidates are evaluated at beginning, mid-way, and end points of the program relative to their attainment of skills included in the standards. Components of the program are designed to prepare future teachers to achieve a high level of performance in each of the teaching skill areas. Skills are demonstrated through university classroom projects; field placement; student teaching, including cooperating teacher observations and conferences and supervising teacher observations and conferences; final projects; examinations; and portfolio development and review. Following each of the Kentucky New Teacher Standards identified below is a description of selected examples of ways the program enhances and assesses candidate performance aligned with the respective standards. Courses that contain these activities and assessments are noted at the end of each narrative. Standard 1: Designs/Plans Instruction The teacher designs/plans instruction and learning climates that develop student abilities to use communication skills, apply core concepts, become self-sufficient individuals, become responsible team members, think and solve problems, and integrate knowledge. 17 Candidates are taught to plan instruction focusing on academic standards as evidenced by inclusion of the standard(s) in the written plan. Lesson plans prepared in university classes and in school classrooms include academic expectations for the lesson. Lesson plans are evaluated based upon their potential for motivating and actively involving diverse learners and emphasis upon higher level thinking skills. Lessons are evaluated based upon developmental level appropriateness, creating an environment for learning to occur, and the consistency of assessment/evaluation strategies used to assess the types of learning included in the lesson. During student teaching, supervisors observe these ideas being put into practice and discuss with the student teacher how improvements can be made in their teaching. (EDC 634, EDC 730, EDC 777) Standard 2: Creates/Maintains Learning Climates The teacher creates a learning climate that supports the development of student abilities to use communication skills, apply core concepts, become self-sufficient individuals, become responsible team members, think and solve problems, and integrate knowledge. Candidates demonstrate a commitment to challenging each student and providing a supportive environment for working and learning. The core content of the MIC Program includes major strands or themes in classroom management and multicultural issues. Candidates select and apply (or modify) one of a number of models for classroom management and prepare a written approach to managing students and classroom in effective ways. Candidates research and incorporate learning activities that appeal to students from a variety of learning, socio-economic, cultural, ethnic, gender, and physical ability backgrounds. Candidates are expected to demonstrate high levels of sensitivity to differences among students and maintain high expectations of all students. Lesson plans include strategies for addressing instruction for students with special needs in the classroom. Cooperating and supervising teachers observe candidate performance in these areas. (EDC 634, EDP 658, EDC 730, EDC 777) Standard 3: Implements/Manages Instruction The teacher introduces/implements/manages instruction that develops student abilities to use communication skills, apply core concepts, become self-sufficient individuals, become responsible team members, think and solve problems, and integrate knowledge. In the science methods course, candidates examine research and experience findings related to the use of effective questioning strategies. Candidates read and discuss a handout (Rowe and others) containing recommendations for effective questioning in the classroom. During student teaching, candidates’ questions are analyzed by the supervising teacher. Recommendations for improvement are made, and a refocusing activity occurs using the methods handout on recommendations. (EDC 634) Candidates are expected to use a variety of modes of instruction in lesson planning and actual teaching. A variety of teaching methods is used to build interest in the science concepts and skills as well as to accommodate a wide range of diversity among learners. Multiple teaching methods are modeled for future teachers in program classes. The field of science lends itself to a variety of modes of instruction. Activities encouraging an understanding of the nature of the scientific enterprise are encouraged and expected. In particular, scientific inquiry is a major theme in the science methods course and in the student teaching experience. Candidates are observed relative to their application of scientific principles to everyday life experiences and are guided toward increased sensitive to the needs and feelings of students. Student teachers provide feedback to students in an effort to guide their learning and help correct misconceptions students may be forming. A major emphasis in class is the discussion of observations and inferences made during exploration and laboratory experiences. (EDC 634, EDC 730, EDC 777, EDC 746) Standard 4: Assesses and Communicates Learning Results The teacher assesses learning and communicates results to students and others with respect to student abilities to use communication skills, apply core concepts, become self-sufficient individuals, become responsible team members, think and solve problems, and integrate knowledge. Candidates embed assessments of daily instruction in lesson planning and make judgments regarding the effectiveness of the instructional strategies used in the classroom. Performance assessment receives considerable emphasis in special (content specific) methods classes. Candidates practice developing innovate and effective assessments and incorporate these elements in lesson and unit planning. Lesson plans and classroom tests are examined to ensure that multiple forms of assessment and evaluation are included in the lesson and classroom. (EDC 634, EDC 730, EDC 777, EDC 746) 18 Standard 5: Reflects/Evaluates Teaching/Learning The teacher reflects on and evaluates specific teaching/learning situations and/or programs. Following instruction, candidates are asked to analyze their teaching and reflect upon ways in which instruction might have been more effective. Candidates are asked to reflect based upon their own feelings about the lesson and do so in an informed manner using what they know about learning and instruction. Following classroom observations by supervisors, candidates are asked to identify strengths and limitations of the day’s instruction. After giving candidates an opportunity to think about the effectiveness of their lessons, the supervisor shares his/her written insights regarding the student teacher’s work. Following the conference, student teachers prepare and submit a written response to comments and recommendations offered by the supervisor. (EDC 634, EDC 730, EDC 777, EDC 746) Standard 6: Collaborates with Colleagues/Parents/Others The teacher collaborates with colleagues, parents, and other agencies to design, implement, and support learning programs that develop student abilities to use communication skills, apply core concepts, become self-sufficient individuals, become responsible team members, think and solve problems, and integrate knowledge. Cohort leaders often ask candidates to work with a student from another discipline (or two) in planning and teaching a mini-unit (or lesson) incorporating concepts and skills from each of the disciplines. For example, a history and mathematics teachers taught a short unit incorporating selected history/culture of Egypt with some of the early and very significant mathematics knowledge derived from that culture. Another group of candidates combined a study of radiation with the massive destruction of Hiroshima and its effect upon that society. These activities have helped candidates discover value in collaboration among teachers of different disciplines, i.e., interdisciplinary lesson project. (EDC 730, EDC 777, EDC 746) Standard 7: Engages in Professional Development The teacher evaluates his/her overall performance with respect to modeling and teaching Kentucky's learning goals, refines the skills and processes necessary, and implements a professional development plan. Candidates are expected to participate in a content-specific professional meeting at the regional or state level. For example, science candidates participate in the annual meeting of the Kentucky Science Teachers Association (KSTA) and mathematics teachers participate in the annual meeting of the Kentucky Council of Teachers of Mathematics (KCTM). Candidates plan their agenda from a pre-prepared program, project what they will learn from the experience, participate in sessions, share conference gleanings with fellow candidates, and prepare a written evaluation of the experience and their accomplishments. This fall, science methods candidates are preparing a group presentation in November at KSTA. Participation in the conference supports candidates’ identity with the teaching profession and with professionals in the field. In addition, the activity supports the theme of developing leaders within the profession. In these settings, candidates have an opportunity to meet, and talk with, some of the best teacher leaders in Kentucky. (EDC 634, EDC 746) Standard 8: Knowledge of Content The teacher demonstrates a current and sufficient academic knowledge of certified content areas to develop student knowledge and performance in those areas. Grades in the content major (and support areas) and Praxis examination scores serve as one type of evidence indicating knowledge of the content area. Candidates in mathematics and the sciences at UK tend to have good grades, pass the content-area PRAXIS examinations, and exhibit strong intellectual skills as indicated by GRE scores. Candidates are asked to respond to thought questions/problems from the content area in the program admission interviews. This enables program representatives to get a glimpse of candidates’ abilities and aptitudes toward the content area. Special methods courses provide extended experiences in the special nature of mathematics and/or the sciences. What does it mean to be a mathematician, physicist, biologist, chemist, or earth scientist? What are the “big ideas” of mathematics and/or science? What are the modes of inquiry in the sciences or mathematics? How does one go about the processes of doing science or mathematics? What is really important about the disciplines that must be taught to young people? What content (knowledge, skills, and attitudes) is developmentally appropriate and must be included in the secondary school programs? How can this content be arranged and structured to capture the interests and engagement of high school students? Questions like these constitute a significant part of the special methods courses. Candidates are given many opportunities to read, discuss, demonstrate, and write about these questions. These experiences tend to increase candidates’ 19 understanding of the disciplines and increase their level of excitement, and preparation, for teaching mathematics or science. (EDC 634, EDC 746) Standard 9: Demonstrates Implementation of Technology The teacher uses technology to support instruction; access and manipulate data; enhance professional growth and productivity; communicate and collaborate with colleagues, parents, and the community; and conduct research. Over the past few years, future teachers have entered the teaching profession with much higher levels of computer skills even though there remains a range of abilities exhibited by these candidates. The use of technology is another strand within the MIC Program. Cohort leaders, technology instructors, and special methods instructors stress the importance of these teaching skills. Both within the core program and special methods, candidates are given opportunities to complete technology projects. Projects include spreadsheets, power point presentations, applied use of the internet for data sources, and gathering and interpreting data. Candidates demonstrate their abilities to use technology by preparing lessons using technology. Candidate work demonstrates the use of technology to address interests and abilities of diverse student needs and learning styles. Mathematics and science teachers tend to use the full range of technology applications in teaching; however, gathering and analyzing real data are recurring emphases in many of their applications. (EDC 634, EDC 730, EDC 777) Unit Functional Skills and Dispositions The Science Education Program endorsed the Unit Functional Skills and Dispositions for assessment of professional dispositions expected of its candidates. Following each disposition are selected examples illustrating ways in which the disposition is enhanced and assessed throughout the program. Functional Skill and Disposition 1: Candidates communicate appropriately and effectively. • Communicates orally in formal presentations • Communicates with individuals in small groups in informal settings • Uses nonverbal communication skills • Communicates in writing (reports, essays, letters, memos, emails) Science certification seekers present data to other candidates in methods classes and receive feedback from other members of the class and instructor about the clarity of the presentation. At the end of the program, candidates must formally present an exit portfolio to a group of evaluators. The presenter must clearly communicate their projects to the evaluators. Pre-service science teachers submit several written reports, essays, and reflections to their various supervisors. Supervisors comment upon the content of these writing samples and suggest improvements to the writing. When a candidate’s writing contains multiple serious problems, the science supervisor may schedule additional meetings with the candidate to improve their writing or suggest additional writing resources available from the UK Writing Center. Entry into the program requires the submission of a writing sample. Student teachers are observed in high school classrooms through student teaching and fall placements. Comments about the student’s communication both in whole group and individualized instruction stem from these observations. During post observation conferences, a discussion of the positive and improvement areas noted by the observer follows an observation. The pre-service teachers write a response to the comments arising from the post-observation conference. Functional Skill and Disposition 2: Candidates demonstrate constructive attitudes. • Demonstrates knowledge and command of socio-cultural variables in education • Demonstrates constructive attitudes toward children, youth, parents, and the community • Demonstrates awareness and acceptance of diversity in educational settings In the common core courses for the MIC program, candidates write several papers demonstrating knowledge of sociocultural variables in education and diversity in educational settings. A written management plan includes detailed descriptions of rules, policies, and practices for a science classroom. The plan incorporates how the pre-service teacher intends to address specific problems presented by diversity (e.g., ADD, racial characteristics, non-native English speakers, 20 gender bias). A multicultural assignment details candidates’ increasing awareness of other cultures and knowledge of these cultures. In methods courses, candidates discuss various forms of interaction they experienced with students, parents, and the community. Discussions of possible diplomatic solutions to problem interactions ensue. Functional Skill and Disposition 3: Candidates demonstrate ability to conceptualize key subject matter ideas and relationships. • Correctly states key subject matter ideas • Explains key subject matter ideas • Tailors key subject matter ideas to diverse populations • Addresses misconceptions in key subject matter ideas • Identifies real life examples to enhance student learning Secondary science education majors develop science units including state and national standards. The units are reviewed and commented upon for content both extraneous and omitted information. The unit assessment includes evaluation of how candidates plan for not only the typical student but the atypical student within the classroom. Appropriate lesson modifications are evaluated. Classroom observations include notes about the candidate’s knowledge of the subject and their facilitation of instruction with the entire classroom. As the pre-service teacher facilitates questioning in a classroom, observers note how well the student teacher acknowledges misconceptions and the method(s) student teachers use to address the misconception. Student teachers are also encouraged to bring pertinent examples of the instructional objectives from everyday experiences. These skills are evaluated by the supervisor and the cooperating teacher on observation forms and these evaluations are shared with the student teacher in post-observation conferences. Functional Skill and Disposition 4: Candidates interact appropriately and effectively with diverse groups of colleagues, administrators, students, and parents in educational settings. • Demonstrates acceptable educator behavior in diverse educational settings • Demonstrates adaptability in reflecting on self in relation to diverse groups Student teacher observations by both the cooperating teachers and the university supervisor comment upon the student teacher’s professional behavior in the school setting. In the fall semester, cohort groups engage in discussions surrounding professional behavior. All student teachers submit reflective pieces via e-mail about their classroom performance after all formal observations. These reflections comment upon the candidate’s opinion about the post conference and methods the individual could use to address the problems in future lessons. Functional Skill and Disposition 5: Candidates demonstrate a commitment to professional ethics and behavior. • Demonstrates understanding of the Kentucky School Personnel Code of Ethics • Complies with all legal requirements required of educators in a knowledgeable and timely manner • Demonstrates understanding of ethical issues related to own professional certification area Candidates read and sign the Kentucky Code of Ethics in their cohort groups. They read passages from The Ethics of Teaching (Strike & Soltis, 2004, 4th Ed., Teachers College Press) and engage in classroom discussions. In the science methods courses, candidates learn of the additional responsibilities associated with laboratory safety. During student teaching, cooperating teachers and supervisors discuss possible safety problems associated with the laboratory experiences. Observation reports document candidates’ compliance or needs for improvement in science laboratory safety. 21 Unit Technology Standards Technology Standard 1: Candidates integrate media and technology into instruction. Candidates pursing certification in science education develop lesson plans involving the use of technology and media into the instruction of the single topic unit. In the course, candidates use probes connected to computers, graphical software, web questions, spreadsheets, e-mail, and PowerPoint presentations to collect data and disseminate their findings. Methods Candidates in methods courses experience using many different forms of media in the science classroom including, but not limited to compasses, rulers, protractors, sand kits, laboratory glassware, safety devises and butterfly nets. From these classroom experiences, candidates integrate these uses and others into unit plans intended for use during student teaching. Technology Standard 2: Candidates utilize multiple technology applications to support student learning. Science education majors usually have previous experiences using probes and graphing software from the physics laboratories. In the methods course, candidates expand their experiences with technology and discuss how using multiple applications of technology supports the learning of a diverse student population. During the science methods course, instructors encourage including technology in the unit lessons plans. During student teaching, the supervisor must observe the student teacher using technology with the class. The program prefers to observe the student teacher facilitating student use of technology. Technology Standard 3: Candidates select appropriate technology to enhance instruction. In the methods course, candidates develop a unit plan for a specific topic in science. Within this plan, instructors encouraged the inclusion of technology. During student teaching, the student teacher’s supervisor conferences with him/her after the technology lesson about the selected technology application. The supervisor raises questions about the candidate’s selection of technology and how the use of technology improved or hindered the lesson. On the observation form, filled out online, the lesson evaluation includes the use of technology in the classroom. Technology Standard 4: Candidates integrate student use of technology into instruction. Student teachers are observed by their supervisor and their cooperating teacher. The supervisor encourages the use of technology with the students, and the cooperating teacher supports the candidate’s use of technology with high school students. At the end of a formal observation, the student teacher’s use of technology in instruction is evaluated within the context of the lesson. At the conclusion of the student teaching semester, an overall evaluation of student teacher’s technology use with high school students is determined from the recommendations of the cooperating teacher and the supervisor as well as the written observation forms submitted over the semester. Technology Standard 5: Candidates address special learning needs through technology. On lesson plans, candidates comment how the technology addresses a special learning need. This special learning need includes clarification of a relationship between two variables or accommodation of an individual education plan in the classroom. Technology Standard 6: Candidates promote ethical and legal use of technology disciplines. Pre-service teachers follow ethical and legal uses of technology. Candidates cite electronic sources when used for a lesson plan, presentation, or paper. Instructors explain the difference between a single user license and a site license for software. 22 National Science Teachers Association Standards Alignment of the science education curriculum and experiences with the standards of the National Science Teachers Association (NSTA) is depicted in the following matrices. The first matrix identifies competency requirements expected of all secondary science teachers. This matrix is followed by matrices identifying competencies expected for biology, chemistry, earth science, and physics, respectively. Integration of NSTA Standards in the Science Education Program Competency Requirements for All Science Teachers Competency – All secondary science teachers should be prepared to lead students to understand the unifying concepts of science which include the following: 1. Multiple ways we organize our perceptions of the world and how systems organize the studies and knowledge of science. 2. Nature of scientific evidence and the use of models for explanation. 3. Measurement as a way of knowing and organizing observations of constancy and change. Required Courses EDC 634 Science Pedagogy in Secondary School (through campus and schoolbased experiences, candidates learn how to engage young people in learning science and how to make decisions about planning instruction developing assessment based upon a sound knowledge base for applying content materials and methods [including educational technology] appropriate for high school students) – [the role of science and scientists; using process skills as ways of gathering and interpreting observations/data; problem solving] BIO 150, Principles of Biology I (develop appreciation of biological principles necessary to explore life at the cellular and molecular level; similarities and differences in structure and function of simple and complex cells covered along with theories on the origin and evolution of biological systems) BIO 151, Principles of Biology Laboratory I (introductory laboratory in which biological systems are investigated at the cellular and molecular levels) EDC 634 Science Pedagogy in Secondary School (through campus and schoolbased experiences, candidates learn how to engage young people in learning science and how to make decisions about planning instruction developing assessment based upon a sound knowledge base for applying content materials and methods [including educational technology] appropriate for high school students) – [analysis of questions that science can and cannot suggest an answer focusing on the methods of science as ways of generating new knowledge – students use a classroom observation to generate, test and refine a functional model that helps explain and make predictions related to observations] CHE 105, General College Chemistry I (basic atomic structure, bonding, reactions, periodicity, thermodynamics, intermolecular forces, quantum theory, hybridization, mass relationships in chemical reactions, gases) GLY 220, Principles of Physical Geology (integrated course in physical geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps; student report on current, newsworthy, geology topic; surface and ground water; global climate change) EDC 634 Science Pedagogy in Secondary School (through campus and schoolbased experiences, candidates learn how to engage young people in learning science and how to make decisions about planning instruction developing assessment based upon a sound knowledge base for applying content materials and methods [including educational technology] appropriate for high school students) – [numerous opportunities are provided for students to make measurements analyze data and make decisions based upon analyses of measurements – electronic sensors, interface devices and computer software are frequently included in making measurements and analyzing data] 23 4. Evolution of natural systems and factors that result in evolution or equilibrium. 5. Interrelationships of form, function, and behaviors in living and nonliving systems. BIO 151, Principles of Biology Laboratory I (introductory laboratory in which biological systems are investigated at the cellular and molecular levels) CHE 115, General Chemistry Laboratory (separations, synthesis, measurements, mathematical applications, basic laboratory skills, safety) GLY 220, Principles of Physical Geology (integrated course in physical geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps; student report on current, newsworthy, geology topic; surface and ground water; global climate change) EDC 634 Science Pedagogy in Secondary School (through campus and schoolbased experiences, candidates learn how to engage young people in learning science and how to make decisions about planning instruction developing assessment based upon a sound knowledge base for applying content materials and methods [including educational technology] appropriate for high school students) – [evolution is presented and discussed as a major unifying theme in the sciences; arguments are identified regarding rationale for teaching evolutionary theory in science classes in lieu of “creationist theories” or theories of “intelligent design”; teachers are encouraged to be sensitive to the beliefs of young adults while teaching scientific knowledge and the role of scientific theories] BIO 150, Principles of Biology I (develop appreciation of biological principles necessary to explore life at the cellular and molecular level; similarities and differences in structure and function of simple and complex cells covered along with theories on the origin and evolution of biological systems) BIO 151, Principles of Biology Laboratory I (introductory laboratory in which biological systems are investigated at the cellular and molecular levels) GLY 220, Principles of Physical Geology (integrated course in physical geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps; student report on current, newsworthy, geology topic; surface and ground water; global climate change) CHE 107, General College Chemistry II (kinetics, acid-base, thermodynamics, electrochemistry, molecular geometry, hybridization, intermolecular forces, physical properties of solutions, equilibrium) EDC 634 Science Pedagogy in Secondary School (through campus and schoolbased experiences, candidates learn how to engage young people in learning science and how to make decisions about planning instruction developing assessment based upon a sound knowledge base for applying content materials and methods [including educational technology] appropriate for high school students) – [students participate in a field experience (normally a stream study); observations are made of organisms in the context of their natural environments focusing on inferring relationships between form and function] BIO 150, Principles of Biology I (develop appreciation of biological principles necessary to explore life at the cellular and molecular level; similarities and differences in structure and function of simple and complex cells covered along with theories on the origin and evolution of biological systems) GLY 220, Principles of Physical Geology (integrated course in physical geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps; student report on current, newsworthy, geology topic; surface and ground water; global climate change) 24 CHE 105, General College Chemistry I (basic atomic structure, bonding, reactions, periodicity, thermodynamics, intermolecular forces, quantum theory, hybridization, mass relationships in chemical reactions, gases) CHE 107, General College Chemistry II (kinetics, acid-base, thermodynamics, electrochemistry, molecular geometry, hybridization, intermolecular forces, physical properties of solutions, equilibrium) 25 NSTA Science Content Requirements for Biology: Analysis Tables I (Core Competencies), II (Advanced Competencies), and III (Supporting Competencies) Table I: Biology – Core Competencies A. Core Competencies – All teachers of biology should be prepared to lead students to understand the unifying concepts required of all teachers of Required Courses science, and should in addition be prepared to lead students to understand the following: 1. Life processes in living systems BIO 150, Principles of Biology I (develop appreciation of biological principles including organization of matter and necessary to explore life at the cellular and molecular level; similarities and energy. differences in structure and function of simple and complex cells covered along with theories on the origin and evolution of biological systems) BIO 151, Principles of Biology Laboratory I (introductory laboratory in which biological systems are investigated at the cellular and molecular levels) BIO 152, Principles of Biology II (designed to develop understanding and appreciation for diverse forms of plant and animal life, and their relationships to each other and to their environment; structure and function relationships will be explored at many levels of organization: cell tissue, organ, organism, population, and community) BIO 153, Principle of Biology Laboratory II (introductory lab course in which biological systems are investigated organismal, population and community levels) BIO 315, Introduction to Cell Biology (structure and function of cells; emphasis placed upon the ultra structure of cell organelles in plants and animals as a framework for understanding compartmentalized nature of cell activity) BCH 401G, Fundamentals of Biochemistry (Descriptive chemistry of amino acids and proteins, carbohydrates, lipids, nucleic acids. Discussion of structure and function; metabolism, and bioenergetics; and biological information flow.) 2. Similarities and differences among BIO 150, Principles of Biology I (develop appreciation of biological principles animals, plants, fungi, microorganisms, necessary to explore life at the cellular and molecular level; similarities and and viruses differences in structure and function of simple and complex cells covered along with theories on the origin and evolution of biological systems) BIO 151, Principles of Biology Laboratory I (introductory laboratory in which biological systems are investigated at the cellular and molecular levels) BIO 152, Principles of Biology II (designed to develop understanding and appreciation for diverse forms of plant and animal life, and their relationships to each other and to their environment; structure and function relationships will be explored at many levels of organization: cell tissue, organ, organism, population, and community) BIO 153, Principle of Biology Laboratory II (introductory lab course in which biological systems are investigated at organismal, population and community levels) 3. Principles and practices of biological BIO 150, Principles of Biology I (develop appreciation of biological principles classification necessary to explore life at the cellular and molecular level; similarities and differences in structure and function of simple and complex cells covered along with theories on the origin and evolution of biological systems) BIO 151, Principles of Biology Laboratory I (introductory laboratory in which biological systems are investigated at the cellular and molecular levels) BIO 152, Principles of Biology II (designed to develop understanding and appreciation for diverse forms of plant and animal life, and their relationships to each other and to their environment; structure and function relationships will be explored at many levels of organization: cell tissue, organ, organism, population, and community) 26 4. Scientific theory and principles of biological evolution 5. Ecological systems including the interrelationships and dependencies of organisms with each other and their environments 6. Population dynamics and the impact of population on its environment 7. General concepts of genetics and heredity 8. Organization and functions of cells and multicellular systems 9. Behavior of organisms and their relationships to social systems BIO 153, Principle of Biology Laboratory II (introductory lab course in which biological systems are investigated at organismal, population and community levels) Biology 351, Plant Kingdom [Upper Level Botany Course – most commonly selected] (evolutionary survey of morphology, taxonomy, life histories, and biological relationships of all plant groups comprising plant kingdom) BIO 150, Principles of Biology I (develop appreciation of biological principles necessary to explore life at the cellular and molecular level; similarities and differences in structure and function of simple and complex cells covered along with theories on the origin and evolution of biological systems) Biology 351, Plant Kingdom [Upper Level Botany Course – most commonly selected] (evolutionary survey of morphology, taxonomy, life histories, and biological relationships of all plant groups comprising plant kingdom) BIO 152, Principles of Biology II (designed to develop understanding and appreciation for diverse forms of plant and animal life, and their relationships to each other and to their environment; structure and function relationships will be explored at many levels of organization: cell tissue, organ, organism, population, and community) BIO 325, Introductory Ecology (basic concepts of ecology; topics include adaptations of organisms to environment; factors that influence distribution and abundance of species; population structure, dynamics, and regulation; community development – succession- , structure and function; food webs, energy flow, and nutrient cycling) BIO 152, Principles of Biology II (designed to develop understanding and appreciation for diverse forms of plant and animal life, and their relationships to each other and to their environment; structure and function relationships will be explored at many levels of organization: cell tissue, organ, organism, population, and community) BIO 153, Principle of Biology Laboratory II (introductory lab course in which biological systems are investigated at organismal, population and community levels) BIO 325, Introductory Ecology (basic concepts of ecology; topics include adaptations of organisms to environment; factors that influence distribution and abundance of species; population structure, dynamics, and regulation; community development – succession- , structure and function; food webs, energy flow, and nutrient cycling) BIO 304, Principles of Genetics (study of the physical and chemical aspects of the genetic material and their relationship to the expression and inheritance of the phenotype) BIO 150, Principles of Biology I (develop appreciation of biological principles necessary to explore life at the cellular and molecular level; similarities and differences in structure and function of simple and complex cells covered along with theories on the origin and evolution of biological systems) BIO 152, Principles of Biology II (designed to develop understanding and appreciation for diverse forms of plant and animal life, and their relationships to each other and to their environment; structure and function relationships will be explored at many levels of organization: cell tissue, organ, organism, population, and community) BIO 315, Introduction to Cell Biology (structure and function of cells; emphasis placed upon the ultra structure of cell organelles in plants and animals as a framework for understanding compartmentalized nature of cell activity) BIO 325, Introductory Ecology (basic concepts of ecology; topics include adaptations of organisms to environment; factors that influence distribution and abundance of species; population structure, dynamics, and regulation; community development – succession- , structure and function; food webs, energy flow, and nutrient cycling) BIO 350, Animal Physiology (introduction basic principles of animal physiology; elementary discussion of major vertebrae organ systems including 27 10. Regulation of biological systems including homeostatic mechanisms 11. Fundamental processes of modeling and investigating in the biological sciences 12. Applications of biology in environmental quality and in personal and community health nutrition, metabolism, respiration, circulation, excretion, muscle contraction, peripheral and central nervous system, and endocrine function; emphasizing homeostasis; behavior; energy expenditure and energetic costs) BIO 325, Introductory Ecology (basic concepts of ecology; topics include adaptations of organisms to environment; factors that influence distribution and abundance of species; population structure, dynamics, and regulation; community development – succession- , structure and function; food webs, energy flow, and nutrient cycling) BIO 350, Animal Physiology (introduction basic principles of animal physiology; elementary discussion of major vertebrae organ systems including nutrition, metabolism, respiration, circulation, excretion, muscle contraction, peripheral and central nervous system, and endocrine function; emphasizing homeostasis; behavior; energy expenditure and energetic costs) BIO 151, Principles of Biology Laboratory I (introductory laboratory in which biological systems are investigated at the cellular and molecular levels) BIO 153, Principle of Biology Laboratory II (introductory lab course in which biological systems are investigated at organismal, population and community levels) BIO 152, Principles of Biology II (designed to develop understanding and appreciation for diverse forms of plant and animal life, and their relationships to each other and to their environment; structure and function relationships will be explored at many levels of organization: cell tissue, organ, organism, population, and community) BIO 325, Introductory Ecology (basic concepts of ecology; topics include adaptations of organisms to environment; factors that influence distribution and abundance of species; population structure, dynamics, and regulation; community development – succession- , structure and function; food webs, energy flow, and nutrient cycling) BIO 350, Animal Physiology (introduction basic principles of animal physiology; elementary discussion of major vertebrae organ systems including nutrition, metabolism, respiration, circulation, excretion, muscle contraction, peripheral and central nervous system, and endocrine function; emphasizing homeostasis; behavior; energy expenditure and energetic costs) Table II: Biology – Advanced Competencies B. Advanced Competencies – In addition to core competencies, teachers of biology as a primary field Required Courses should be prepared to effectively lead students to understand: 13. Bioenergetics and major BIO 315, Introduction to Cell Biology (structure and function of cells; biochemical pathways emphasis placed upon the ultra structure of cell organelles in plants and animals as a framework for understanding compartmentalized nature of cell activity) BIO 350, Animal Physiology (introduction basic principles of animal physiology; elementary discussion of major vertebrae organ systems including nutrition, metabolism, respiration, circulation, excretion, muscle contraction, peripheral and central nervous system, and endocrine function; emphasizing homeostasis; behavior; energy expenditure and energetic costs) BCH 401G, Fundamentals of Biochemistry (Descriptive chemistry of amino acids and proteins, carbohydrates, lipids, nucleic acids. Discussion of structure and function; metabolism, and bioenergetics; and biological information flow.) 14. Biochemical interactions of BIO 152, Principles of Biology II (designed to develop understanding and organisms with their environments appreciation for diverse forms of plant and animal life, and their relationships to each other and to their environment; structure and function relationships will be explored at many levels of organization: cell tissue, organ, organism, population, and community) BIO 325, Introductory Ecology (basic concepts of ecology; topics include 28 15. Molecular genetics and heredity and mechanisms of genetic modification 16. Molecular basis for evolutionary theory and classification 17. Causes, characteristics, and avoidance of viral, bacterial, and parasitic diseases 18. Issues related to living systems such as genetic modification, uses of biotechnology, cloning, and pollution from farming 19. Historical development and perspectives in biology including contributions of significant figures and underrepresented groups, and the evolution of theories in biology 20. How to design, conduct, and report research in biology adaptations of organisms to environment; factors that influence distribution and abundance of species; population structure, dynamics, and regulation; community development – succession- , structure and function; food webs, energy flow, and nutrient cycling) BIO 304, Principles of Genetics (study of the physical and chemical aspects of the genetic material and their relationship to the expression and inheritance of the phenotype) BIO 150, Principles of Biology I (develop appreciation of biological principles necessary to explore life at the cellular and molecular level; similarities and differences in structure and function of simple and complex cells covered along with theories on the origin and evolution of biological systems) BIO 151, Principles of Biology Laboratory I (introductory laboratory in which biological systems are investigated at the cellular and molecular levels) Biology 351, Plant Kingdom [Upper Level Botany Course – most commonly selected] (evolutionary survey of morphology, taxonomy, life histories, and biological relationships of all plant groups comprising plant kingdom) BIO 151, Principles of Biology Laboratory I (introductory laboratory in which biological systems are investigated at the cellular and molecular levels) BIO 153, Principle of Biology Laboratory II (introductory lab course in which biological systems are investigated at organismal, population and community levels) 21. Applications of biology and biotechnology in society, business, industry, and health fields Table III: Biology – Supporting Competencies C. Supporting Competencies – All teachers of biology should also be prepared to effectively apply Required Courses concepts from other sciences and mathematics to the teaching of biology including basic concepts of: 22. Chemistry, including general CHE 105, General College Chemistry I (basic atomic structure, bonding, chemistry and biochemistry with basic reactions, periodicity, thermodynamics, intermolecular forces, quantum theory, laboratory techniques. hybridization, mass relationships in chemical reactions, gases) CHE 107, General College Chemistry II (kinetics, acid-base, thermodynamics, electrochemistry, molecular geometry, hybridization, intermolecular forces, physical properties of solutions, equilibrium) CHE 115, General Chemistry Laboratory (separations, synthesis, measurements, mathematical applications, basic laboratory skills, safety) CHE 230, General Organic Chemistry I (content can be classified as structure, reactivity, and synthesis;. how atoms are joined together in organic compounds, how structure affects bulk properties, how scientists can gain information about the structure of unknown organic compounds, and how organic compounds are transformed into other organic compounds) 29 23. Physics including light, sound, optics, electricity, energy and order, magnetism, and thermodynamics. 24. Earth and space sciences including energy and geochemical cycles, climate, oceans, weather, natural resources, and changes in the Earth. 25. Mathematics, including probability and statistics CHE 231, Organic Chemistry Laboratory I (recrystallization, solvent extraction, fractional distillation, thin layer chromatography, Grignard reaction, multi-step synthesis) CHE 232, General Organic Chemistry II (complex reactions and systems; polyenes and aromatic compounds; highly functionalized systems such as alcohols, amines, amino acids, carbohydrates) CHE 233, Organic Chemistry Laboratory II (infrared spectroscopy; classification tests and derivatives; GC/MS [gas chromatography and mass spectrometry]; identification of compounds; separations) BCH 401G, Fundamentals of Biochemistry [Recommended] (Descriptive chemistry of amino acids and proteins, carbohydrates, lipids, nucleic acids. Discussion of structure and function; metabolism, and bioenergetics; and biological information flow.) PHY 211, General Physics (survey of classical and modern physics focusing on the motion of solids and fluids as governed by Newton’s laws and by the conservation law of energy, momentum, and angular momentum) PHY 213, General Physics (electrostatics, dc circuits, magnetism, Maxwell’s equations, electromagnetic radiation, light, and modern physics) GLY 220, Principles of Physical Geology (integrated course in physical geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps; student report on current, newsworthy, geology topic; surface and ground water; global climate change) MA 123, Elementary Calculus (introduction to differential and integral calculus with applications to business and biological and physical sciences) or MA 132, Calculus for Life Sciences (introduction to integral calculus, integration of logarithmic and exponential functions; application to life sciences including biochemical rates, reactions, and radioactive decay; introduction to biological models and their associated differential equations) or MA 113, Calculus I (course in one variable calculus, including topics from analytic geometry; derivatives and integrals of elementary functions – including trigonometric functions – with applications) 30 NSTA Science Content Requirements for Chemistry: Analysis Tables I (Core Competencies), II (Advanced Competencies), and III (Supporting Competencies) Table I: Chemistry – Core Competencies A. Core Competencies – All teachers of chemistry should be prepared to lead students to understand the unifying concepts required of all Required Courses teachers of science, and should in addition be prepared to lead students to understand: 1. Fundamental structures of atoms and CHE 105, General College Chemistry I (basic atomic structure, bonding, molecules reactions, periodicity, thermodynamics, intermolecular forces, quantum theory, hybridization, mass relationships in chemical reactions, gases) CHE 107, General College Chemistry II (kinetics, acid-base, thermodynamics, electrochemistry, molecular geometry, hybridization, intermolecular forces, physical properties of solutions, equilibrium) CHE 115, General Chemistry Laboratory (separations, synthesis, measurements, mathematical applications, basic laboratory skills, safety) 2. Basic principles of ionic, covalent, CHE 105, General College Chemistry I (basic atomic structure, bonding, and metallic bonding reactions, periodicity, thermodynamics, intermolecular forces, quantum theory, hybridization, mass relationships in chemical reactions, gases) 3. Physical and chemical properties CHE 105, General College Chemistry I (basic atomic structure, bonding, and classification of elements reactions, periodicity, thermodynamics, intermolecular forces, quantum theory, including periodicity hybridization, mass relationships in chemical reactions, gases) 4. Chemical kinetics and CHE 105, General College Chemistry I (basic atomic structure, bonding, thermodynamics reactions, periodicity, thermodynamics, intermolecular forces, quantum theory, hybridization, mass relationships in chemical reactions, gases) CHE 107, General College Chemistry II (kinetics, acid-base, thermodynamics, electrochemistry, molecular geometry, hybridization, intermolecular forces, physical properties of solutions, equilibrium) CHE 440G, Introductory Physical Chemistry (introduction to the laws of thermodynamics, the thermodynamics functions and their application to phase equilibria, chemical equilibria, solutions and electrochemistry; chemical kinetics, including rate laws, reaction mechanism; Arrhenius, collision, and activated complex theories, and catalysis; quantum theory including an elementary introduction to spectroscopy) 5. Principles of electrochemistry CHE 107, General College Chemistry II (kinetics, acid-base, thermodynamics, electrochemistry, molecular geometry, hybridization, intermolecular forces, physical properties of solutions, equilibrium) CHE 226, Analytical Chemistry (stoichiometry, concentration, experimental error, statistical analysis, gravimetric analysis, chemical equilibrium, electrolyte effects, equilibrium in complex systems, acid-base titrations, spectrochemical analysis, electrochemistry, electrode potentials, oxidation and reduction titrations, potentiometry) CHE 440G, Introductory Physical Chemistry (introduction to the laws of thermodynamics, the thermodynamics functions and their application to phase equilibria, chemical equilibria, solutions and electrochemistry; chemical kinetics, including rate laws, reaction mechanism; Arrhenius, collision, and activated complex theories, and catalysis; quantum theory including an elementary introduction to spectroscopy) 6. Mole concept, stoichiometry, and CHE 105, General College Chemistry I (basic atomic structure, bonding, laws of composition reactions, periodicity, thermodynamics, intermolecular forces, quantum theory, hybridization, mass relationships in chemical reactions, gases) CHE 107, General College Chemistry II (kinetics, acid-base, thermodynamics, electrochemistry, molecular geometry, hybridization, intermolecular forces, physical properties of solutions, equilibrium) 31 CHE 226, Analytical Chemistry (stoichiometry, concentration, experimental error, statistical analysis, gravimetric analysis, chemical equilibrium, electrolyte effects, equilibrium in complex systems, acid-base titrations, spectrochemical analysis, electrochemistry, electrode potentials, oxidation and reduction titrations, potentiometry) 7. Transition elements and coordination compounds 8. Acids and bases, oxidation-reduction chemistry, and solutions 9. Fundamental biochemistry 10. Functional and polyfunctional group chemistry 11. Environmental and atmospheric chemistry 12. Fundamental processes of investigating in chemistry 13. Applications of chemistry in personal and community health and environmental quality CHE 107, General College Chemistry II (kinetics, acid-base, thermodynamics, electrochemistry, molecular geometry, hybridization, intermolecular forces, physical properties of solutions, equilibrium) CHE 226, Analytical Chemistry (stoichiometry, concentration, experimental error, statistical analysis, gravimetric analysis, chemical equilibrium, electrolyte effects, equilibrium in complex systems, acid-base titrations, spectrochemical analysis, electrochemistry, electrode potentials, oxidation and reduction titrations, potentiometry) BCH 401G, Fundamentals of Biochemistry (Descriptive chemistry of amino acids and proteins, carbohydrates, lipids, nucleic acids. Discussion of structure and function; metabolism, and bioenergetics; and biological information flow.) CHE 230, General Organic Chemistry I (content can be classified as structure, reactivity, and synthesis;. how atoms are joined together in organic compounds, how structure affects bulk properties, how scientists can gain information about the structure of unknown organic compounds, and how organic compounds are transformed into other organic compounds) CHE 232, General Organic Chemistry II (complex reactions and systems; polyenes and aromatic compounds; highly functionalized systems such as alcohols, amines, amino acids, carbohydrates) CHE 115, General Chemistry Laboratory (separations, synthesis, measurements, mathematical applications, basic laboratory skills, safety) CHE 226, Analytical Chemistry (stoichiometry, concentration, experimental error, statistical analysis, gravimetric analysis, chemical equilibrium, electrolyte effects, equilibrium in complex systems, acid-base titrations, spectrochemical analysis, electrochemistry, electrode potentials, oxidation and reduction titrations, potentiometry) CHE 231, Organic Chemistry Laboratory I (recrystallization, solvent extraction, fractional distillation, thin layer chromatography, Grignard reaction, multi-step synthesis) CHE 233, Organic Chemistry Laboratory II (infrared spectroscopy; classification tests and derivatives; GC/MS [gas chromatography and mass spectrometry]; identification of compounds; separations) CHE 105, General College Chemistry I (basic atomic structure, bonding, reactions, periodicity, thermodynamics, intermolecular forces) – Integrated in multiple chapters in the course: Chang, Chapter 3, electron microscopy; Chang, Chapter 7, chemical fertilizers; Chang, Chapter 10, fuel values of food and other substances. CHE 107, General College Chemistry II (kinetics, acid-base, thermodynamics, electrochemistry, molecular geometry, hybridization, intermolecular forces, physical properties of solutions, equilibrium) - Chang, Chapter 15, antacids and the pH balance in your stomach; Chang, Chapter 16, maintaining the pH of blood; Chang, Chapter 18, tainted water. Table II: Chemistry – Advanced Competencies B. Advanced Competencies – In addition to the core competencies, teachers of chemistry as a primary field should also be prepared to Required Courses 32 effectively lead students to understand: 14. Molecular orbital theory, aromaticity, metallic and ionic structures, and correlation to properties of matter 15. Superconductors and principles of metallurgy 16. Advanced concepts of chemical kinetics, and thermodynamics 17. Lewis adducts and coordination compounds 18. Solutions, colloids, and colligative properties 19. Major biological compounds and natural products 20. Solvent system concepts including non-aqueous solvents 21. Chemical reactivity and molecular structure including electronic and steric effects CHE 232, General Organic Chemistry II (complex reactions and systems; polyenes and aromatic compounds; highly functionalized systems such as alcohols, amines, amino acids, carbohydrates) CHE 226, Analytical Chemistry (stoichiometry, concentration, experimental error, statistical analysis, gravimetric analysis, chemical equilibrium, electrolyte effects, equilibrium in complex systems, acid-base titrations, spectrochemical analysis, electrochemistry, electrode potentials, oxidation and reduction titrations, potentiometry) CHE 440G, Introductory Physical Chemistry (introduction to the laws of thermodynamics, the thermodynamics functions and their application to phase equilibria, chemical equilibria, solutions and electrochemistry; chemical kinetics, including rate laws, reaction mechanism; Arrhenius, collision, and activated complex theories, and catalysis; quantum theory including an elementary introduction to spectroscopy) CHE 440G, Introductory Physical Chemistry (introduction to the laws of thermodynamics, the thermodynamics functions and their application to phase equilibria, chemical equilibria, solutions and electrochemistry; chemical kinetics, including rate laws, reaction mechanism; Arrhenius, collision, and activated complex theories, and catalysis; quantum theory including an elementary introduction to spectroscopy) CHE 230, General Organic Chemistry I (content can be classified as structure, reactivity, and synthesis;. how atoms are joined together in organic compounds, how structure affects bulk properties, how scientists can gain information about the structure of unknown organic compounds, and how organic compounds are transformed into other organic compounds) CHE 231, Organic Chemistry Laboratory I (recrystallization, solvent extraction, fractional distillation, thin layer chromatography, Grignard reaction, multi-step synthesis) CHE 232, General Organic Chemistry II (complex reactions and systems; polyenes and aromatic compounds; highly functionalized systems such as alcohols, amines, amino acids, carbohydrates) CHE 233, Organic Chemistry Laboratory II (infrared spectroscopy; classification tests and derivatives; GC/MS [gas chromatography and mass spectrometry]; identification of compounds; separations) BCH 401G, Fundamentals of Biochemistry (Descriptive chemistry of amino acids and proteins, carbohydrates, lipids, nucleic acids. Discussion of structure and function; metabolism, and bioenergetics; and biological information flow.) CHE 226, Analytical Chemistry (stoichiometry, concentration, experimental error, statistical analysis, gravimetric analysis, chemical equilibrium, electrolyte effects, equilibrium in complex systems, acid-base titrations, spectrochemical analysis, electrochemistry, electrode potentials, oxidation and reduction titrations, potentiometry) CHE 230, General Organic Chemistry I (content can be classified as structure, reactivity, and synthesis;. how atoms are joined together in organic compounds, how structure affects bulk properties, how scientists can gain information about the structure of unknown organic compounds, and how organic compounds are transformed into other organic compounds) CHE 232, General Organic Chemistry II (complex reactions and systems; polyenes and aromatic compounds; highly functionalized systems such as alcohols, amines, amino acids, carbohydrates) 33 22. Organic synthesis and organic reaction mechanisms 23. Energy flow through chemical systems 24. Issues related to chemistry including ground water pollution, disposal of plastics, and development of alternative fuels 25. Historical development and perspectives in chemistry including contributions of significant figures and underrepresented groups, and the evolution of theories in chemistry 26. How to design, conduct, and report research in chemistry 27. Applications of chemistry and chemical technology in society, business, industry, and health fields BCH 401G, Fundamentals of Biochemistry (Descriptive chemistry of amino acids and proteins, carbohydrates, lipids, nucleic acids. Discussion of structure and function; metabolism, and bioenergetics; and biological information flow.) CHE 230, General Organic Chemistry I (content can be classified as structure, reactivity, and synthesis;. how atoms are joined together in organic compounds, how structure affects bulk properties, how scientists can gain information about the structure of unknown organic compounds, and how organic compounds are transformed into other organic compounds) CHE 231, Organic Chemistry Laboratory I (recrystallization, solvent extraction, fractional distillation, thin layer chromatography, Grignard reaction, multi-step synthesis) CHE 232, General Organic Chemistry II (complex reactions and systems; polyenes and aromatic compounds; highly functionalized systems such as alcohols, amines, amino acids, carbohydrates) CHE 233, Organic Chemistry Laboratory II (infrared spectroscopy; classification tests and derivatives; GC/MS [gas chromatography and mass spectrometry]; identification of compounds; separations) BCH 401G, Fundamentals of Biochemistry (Descriptive chemistry of amino acids and proteins, carbohydrates, lipids, nucleic acids. Discussion of structure and function; metabolism, and bioenergetics; and biological information flow.) CHE 440G, Introductory Physical Chemistry (introduction to the laws of thermodynamics, the thermodynamics functions and their application to phase equilibria, chemical equilibria, solutions and electrochemistry; chemical kinetics, including rate laws, reaction mechanism; Arrhenius, collision, and activated complex theories, and catalysis; quantum theory including an elementary introduction to spectroscopy) BCH 401G, Fundamentals of Biochemistry (Descriptive chemistry of amino acids and proteins, carbohydrates, lipids, nucleic acids. Discussion of structure and function; metabolism, and bioenergetics; and biological information flow.) CHE 105, General College Chemistry I (basic atomic structure, bonding, reactions, periodicity, thermodynamics, intermolecular forces) – Integrated in multiple chapters in the course: Chang, Chapter 8, an undesirable precipitation reaction. CHE 107, General College Chemistry II (kinetics, acid-base, thermodynamics, electrochemistry, molecular geometry, hybridization, intermolecular forces, physical properties of solutions, equilibrium) – Chang, Chapter 12, the killer lake; Chang, Chapter 15, decaying papers; Chang, Chapter 18, tainted water. CHE 105, General College Chemistry I (basic atomic structure, bonding, reactions, periodicity, thermodynamics, intermolecular forces) CHE 107, General College Chemistry II (kinetics, acid-base, thermodynamics, electrochemistry, molecular geometry, hybridization, intermolecular forces, physical properties of solutions, equilibrium) CHE 115, General Chemistry Laboratory (separations, synthesis, measurements, mathematical applications, basic laboratory skills, safety) CHE 231, Organic Chemistry Laboratory I (recrystallization, solvent extraction, fractional distillation, thin layer chromatography, Grignard reaction, multi-step synthesis) CHE 233, Organic Chemistry Laboratory II (infrared spectroscopy; classification tests and derivatives; GC/MS [gas chromatography and mass spectrometry]; identification of compounds; separations) CHE 105, General College Chemistry I (basic atomic structure, bonding, reactions, periodicity, thermodynamics, intermolecular forces) – Integrated in multiple chapters in the course: Chang, Chapter 2, distribution of elements on earth and in living systems; Chang, Chapter 3, laser – the splendid light, Chang, Chapter 3, electron microscopy; Chang, Chapter 6, microwave ovens – dipole 34 moments at work; Chang, Chapter 7, chemical fertilizers; Chang, Chapter 8, breath analyzer; Chang, Chapter 9, scuba diving and the gas laws; Chang, Chapter 10, making snow and inflating a bicycle tire; Chang, Chapter 10, fuel values of food and other substances; Chang, Chapter 11, pressure cookers and ice skating. CHE 107, General College Chemistry II (kinetics, acid-base, thermodynamics, electrochemistry, molecular geometry, hybridization, intermolecular forces, physical properties of solutions, equilibrium) – Chang, Chapter 12, desalination; Chang, Chapter 13, determining the age of the Shroud of Turin; Chang, Chapter 15, antacids and the pH balance in your stomach; Chang, Chapter 16, maintaining the pH of blood. Table III: Chemistry – Supporting Competencies C. Supporting Competencies – All teachers of chemistry should be prepared to effectively apply Required Courses concepts from other sciences and mathematics to the teaching of chemistry including: 28. Biology, including molecular BIO 150, Principles of Biology I (develop appreciation of biological principles biology, bioenergetics, and ecology necessary to explore life at the cellular and molecular level; similarities and differences in structure and function of simple and complex cells covered along with theories on the origin and evolution of biological systems) BIO 151, Principles of Biology Laboratory I (introductory laboratory in which biological systems are investigated at the cellular and molecular levels) 29. Earth science, including GLY 220, Principles of Physical Geology (integrated course in physical geochemistry, geocycles, and geology covering the physical, chemical, and biological processes that combine energetics of earth systems to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps) 30. Physics, including energy, stellar PHY 211, General Physics (survey of classical and modern physics focusing evolution, properties and functions of on the motion of solids and fluids as governed by Newton’s laws and by the waves, motions and forces, electricity, conservation law of energy, momentum, and angular momentum) magnetism PHY 213, General Physics (electrostatics, dc circuits, magnetism, Maxwell’s equations, electromagnetic radiation, light, and modern physics) 31. Mathematical and statistical MA 113, Calculus I (course in one variable calculus, including topics from concepts and skills including statistics analytic geometry; derivatives and integrals of elementary functions – including and the use of differential equations trigonometric functions – with applications) and calculus MA 114, Calculus II (stressing techniques of integration) CHE 226, Analytical Chemistry (stoichiometry, concentration, experimental error, statistical analysis, gravimetric analysis, chemical equilibrium, electrolyte effects, equilibrium in complex systems, acid-base titrations, spectrochemical analysis, electrochemistry, electrode potentials, oxidation and reduction titrations, potentiometry) 35 NSTA Science Content Requirements for the Earth/Space Sciences: Analysis Tables I (Core Competencies), II (Advanced Competencies), and III (Supporting Competencies) Table I: Earth/Space Sciences – Core Competencies A. Core Competencies – All teachers of the Earth and space sciences should be prepared to lead students to understand the unifying concepts Required Courses required of all teachers of science, and should in addition be prepared to lead students to understand: 1. Characteristics of land, atmosphere, GEO 251, Weather and Climate (survey of atmospheric controls associated and ocean systems on Earth with local, regional, and global weather and climate variability; includes fundamental coverage of physics and chemistry of energy, gases, pressure, and moisture, with a goal of promoting understanding of general weather analysis and forecasting, severe storms, atmospheric pollution, descriptive climatology, and global climate change) OR GEO 130, Earth’s Physical Environment (exploring the fundamental characteristics of earth’s physical environment; emphasis placed on identifying interrelationships between atmospheric processes involving energy, pressure, and moisture, weather and climate, and terrestrial processes of vegetative biomes, soils, and landscape formation and change) 2. Properties, measurement, and GLY 220, Principles of Physical Geology (integrated course in physical classification of Earth materials geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps) GLY 230, Fundamentals of Geology I (field and laboratory methods for identification and description of rocks and minerals with emphasis on sedimentary rocks and rock forming minerals; field study of geologic structures; interpretation of geologic maps) GLY 235, Fundamentals of Geology II (laboratory and field methods for identification and description of rocks and minerals with emphasis on igneous and metamorphic rocks and rock forming minerals; field study of geologic structures; interpretation of geologic maps) 3. Changes in the Earth including land GLY 220, Principles of Physical Geology (integrated course in physical formation and erosion geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps) 4. Geochemical cycles including biotic GLY 220, Principles of Physical Geology (integrated course in physical and abiotic systems geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps) GEO 251, Weather and Climate (survey of atmospheric controls associated with local, regional, and global weather and climate variability; includes fundamental coverage of physics and chemistry of energy, gases, pressure, and moisture, with a goal of promoting understanding of general weather analysis and forecasting, severe storms, atmospheric pollution, descriptive climatology, and global climate change) OR GEO 130, Earth’s Physical Environment (exploring the fundamental characteristics of earth’s physical environment; emphasis placed on identifying interrelationships between atmospheric processes involving energy, pressure, 36 5. Energy flow and transformation in Earth systems 6. Hydrological features of the Earth 7. Patterns and changes in the atmosphere, weather, and climate 8. Origin, evolution, and planetary behaviors of Earth 9. Origin, evolution, and properties of the universe 10. Fundamental processes of investigating in the Earth and space sciences 11. Sources and limits of natural resources 12. Applications of Earth and space sciences to environmental quality and to personal and community health and welfare and moisture, weather and climate, and terrestrial processes of vegetative biomes, soils, and landscape formation and change) GEO 251, Weather and Climate (survey of atmospheric controls associated with local, regional, and global weather and climate variability; includes fundamental coverage of physics and chemistry of energy, gases, pressure, and moisture, with a goal of promoting understanding of general weather analysis and forecasting, severe storms, atmospheric pollution, descriptive climatology, and global climate change) OR GEO 130, Earth’s Physical Environment (exploring the fundamental characteristics of earth’s physical environment; emphasis placed on identifying interrelationships between atmospheric processes involving energy, pressure, and moisture, weather and climate, and terrestrial processes of vegetative biomes, soils, and landscape formation and change) GLY 220, Principles of Physical Geology (integrated course in physical geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps) GEO 251, Weather and Climate (survey of atmospheric controls associated with local, regional, and global weather and climate variability; includes fundamental coverage of physics and chemistry of energy, gases, pressure, and moisture, with a goal of promoting understanding of general weather analysis and forecasting, severe storms, atmospheric pollution, descriptive climatology, and global climate change) OR GEO 130, Earth’s Physical Environment (exploring the fundamental characteristics of earth’s physical environment; emphasis placed on identifying interrelationships between atmospheric processes involving energy, pressure, and moisture, weather and climate, and terrestrial processes of vegetative biomes, soils, and landscape formation and change) AST 191, The Solar System (emphasizing nature, origin, and evolution of planets, satellites, and other objects in the solar system; topics also include historical astronomy, the naked eye phenomenon of the sky and modern solar system discoveries made by spacecraft) AST 192, Stars Galaxies and The Universe [Recommended] (covering the universe outside the solar system; a principal theme is the origin and evolution of stars, galaxies, and the universe at large; topics also include black holes, quasars, and the big bang model of the universe) GLY 220, Principles of Physical Geology (integrated course in physical geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps) GLY 230, Fundamentals of Geology I (field and laboratory methods for identification and description of rocks and minerals with emphasis on sedimentary rocks and rock forming minerals; field study of geologic structures; interpretation of geologic maps) GLY 235, Fundamentals of Geology II (laboratory and field methods for identification and description of rocks and minerals with emphasis on igneous and metamorphic rocks and rock forming minerals; field study of geologic structures; interpretation of geologic maps) GEO 251, Weather and Climate (survey of atmospheric controls associated with local, regional, and global weather and climate variability; includes fundamental coverage of physics and chemistry of energy, gases, pressure, and moisture, with a goal of promoting understanding of general weather analysis 37 and forecasting, severe storms, atmospheric pollution, descriptive climatology, and global climate change) OR GEO 130, Earth’s Physical Environment (exploring the fundamental characteristics of earth’s physical environment; emphasis placed on identifying interrelationships between atmospheric processes involving energy, pressure, and moisture, weather and climate, and terrestrial processes of vegetative biomes, soils, and landscape formation and change) Table II: Earth/Space Sciences – Advanced Competencies B. Advanced Competencies – In addition to the core competencies, teachers of the Earth and space sciences as a primary field should be Required Courses prepared to effectively lead students to understand: 13. Gradual and catastrophic changes GLY 220, Principles of Physical Geology (integrated course in physical in the Earth geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps) 14. Oceans and their relationship to GEO 251, Weather and Climate (survey of atmospheric controls associated changes in atmosphere and climate with local, regional, and global weather and climate variability; includes fundamental coverage of physics and chemistry of energy, gases, pressure, and moisture, with a goal of promoting understanding of general weather analysis and forecasting, severe storms, atmospheric pollution, descriptive climatology, and global climate change) OR GEO 130, Earth’s Physical Environment (exploring the fundamental characteristics of earth’s physical environment; emphasis placed on identifying interrelationships between atmospheric processes involving energy, pressure, and moisture, weather and climate, and terrestrial processes of vegetative biomes, soils, and landscape formation and change) 15. Hydrological cycles and problems of distribution and use of water 16. Dating of the Earth and other GLY 220, Principles of Physical Geology (integrated course in physical objects in the universe geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps) AST 191, The Solar System (emphasizing nature, origin, and evolution of planets, satellites, and other objects in the solar system; topics also include historical astronomy, the naked eye phenomenon of the sky and modern solar system discoveries made by spacecraft) 17. Structures and interactions of GLY 220, Principles of Physical Geology (integrated course in physical energy and matter in the universe geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps) AST 191, The Solar System (emphasizing nature, origin, and evolution of planets, satellites, and other objects in the solar system; topics also include historical astronomy, the naked eye phenomenon of the sky and modern solar system discoveries made by spacecraft) 18. Impact of changes in the Earth on GLY 401G, Invertebrate Paleobiology and Evolution (basic ecologic and the evolution and distribution of living evolutionary framework of common fossil invertebrate taxa; major principles of things paleontology, ecology, systematics and evolution; and the use of fossils in paleoecology and biostratigraphy; laboratory work in classification of common 38 19. Issues related to changes in Earth systems such as global climate change, mine subsidence, and channeling of waterways 20. Historical development and perspectives in the Earth and space sciences, including contributions of significant figures and underrepresented groups, and the evolution of theories in these fields. 21. How to design, conduct, and report research in the Earth and space sciences fossils) OR GLY 360, Mineralogy (study of mineral structure and composition, and mineral classification through crystallographic and crystal chemical technical; laboratory work includes study of minerals via crystallographic, x-ray diffraction, mineral chemical analysis and optical petrographic techniques) GEO 251, Weather and Climate (survey of atmospheric controls associated with local, regional, and global weather and climate variability; includes fundamental coverage of physics and chemistry of energy, gases, pressure, and moisture, with a goal of promoting understanding of general weather analysis and forecasting, severe storms, atmospheric pollution, descriptive climatology, and global climate change) OR GEO 130, Earth’s Physical Environment (exploring the fundamental characteristics of earth’s physical environment; emphasis placed on identifying interrelationships between atmospheric processes involving energy, pressure, and moisture, weather and climate, and terrestrial processes of vegetative biomes, soils, and landscape formation and change) AST 191, The Solar System (emphasizing nature, origin, and evolution of planets, satellites, and other objects in the solar system; topics also include historical astronomy, the naked eye phenomenon of the sky and modern solar system discoveries made by spacecraft) GLY 220, Principles of Physical Geology (integrated course in physical geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps) GLY 220, Principles of Physical Geology (integrated course in physical geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps) GLY 230, Fundamentals of Geology I (field and laboratory methods for identification and description of rocks and minerals with emphasis on sedimentary rocks and rock forming minerals; field study of geologic structures; interpretation of geologic maps) GLY 235, Fundamentals of Geology II (laboratory and field methods for identification and description of rocks and minerals with emphasis on igneous and metamorphic rocks and rock forming minerals; field study of geologic structures; interpretation of geologic maps) 22. Applications of the Earth and space sciences and related technologies in society, business, industry, and health fields Table III: Earth/Space Science – Supporting Competencies C. Supporting Competencies – All teachers of Earth and space sciences should be prepared to effectively apply concepts from other sciences Required Courses and mathematics to the teaching of Earth and space sciences including concepts of: 23. Biology, including evolution, BIO 150, Principles of Biology I (develop appreciation of biological principles ecology, population dynamics, and necessary to explore life at the cellular and molecular level; similarities and flow of energy and materials through differences in structure and function of simple and complex cells covered along Earth systems with theories on the origin and evolution of biological systems) BIO 151, Principles of Biology Laboratory I (introductory laboratory in which 39 24. Chemistry, including broad concepts and basic laboratory techniques of inorganic and organic chemistry, physical chemistry, and biochemistry 25. Physics, including electricity, forces and motion, energy, magnetism, thermodynamics, optics, and sound; as well as basic quantum theory 26. Mathematics, including statistics and probability biological systems are investigated at the cellular and molecular levels) CHE 105, General College Chemistry I (basic atomic structure, bonding, reactions, periodicity, thermodynamics, intermolecular forces, quantum theory, hybridization, mass relationships in chemical reactions, gases) CHE 107, General College Chemistry II (kinetics, acid-base, thermodynamics, electrochemistry, molecular geometry, hybridization, intermolecular forces, physical properties of solutions, equilibrium) CHE 115, General Chemistry Laboratory (separations, synthesis, measurements, mathematical applications, basic laboratory skills, safety) PHY 211, General Physics (survey of classical and modern physics focusing on the motion of solids and fluids as governed by Newton’s laws and by the conservation law of energy, momentum, and angular momentum) PHY 213, General Physics (electrostatics, dc circuits, magnetism, Maxwell’s equations, electromagnetic radiation, light, and modern physics) MA 123, Elementary Calculus (introduction to differential and integral calculus with applications to business and biological and physical sciences) or MA 132, Calculus for Life Sciences (introduction to integral calculus, integration of logarithmic and exponential functions; application to life sciences including biochemical rates, reactions, and radioactive decay; introduction to biological models and their associated differential equations) or MA 113, Calculus I (course in one variable calculus, including topics from analytic geometry; derivatives and integrals of elementary functions – including trigonometric functions – with applications) 40 NSTA Science Content Requirements for Physics: Analysis Tables I (Core Competencies), II (Advanced Competencies), and III (Supporting Competencies) Table I: Physics – Core Competencies A. Core Competencies – All teachers of physics should be prepared to lead students to understand the unifying concepts required of all teachers of Required Courses science, and should in addition be prepared to lead students to understand: 1. Energy, work, and power PHY 231, General University Physics (first part of two-semester survey of classical physics; consequences of the principles of mechanics are developed conceptually, analytically, and quantitatively) PHY 241, General University Physics Laboratory (laboratory course offering experiments in mechanics and heat, framed in a small group environment that requires coordination and team work in the development of a well written lab report) 2. Motion, major forces, and PHY 231, General University Physics (first part of two-semester survey of momentum classical physics; consequences of the principles of mechanics are developed conceptually, analytically, and quantitatively) PHY 241, General University Physics Laboratory (laboratory course offering experiments in mechanics and heat, framed in a small group environment that requires coordination and team work in the development of a well written lab report) 3. Newtonian principles and laws PHY 231, General University Physics (first part of two-semester survey of including engineering applications classical physics; consequences of the principles of mechanics are developed conceptually, analytically, and quantitatively) PHY 241, General University Physics Laboratory (laboratory course offering experiments in mechanics and heat, framed in a small group environment that requires coordination and team work in the development of a well written lab report) 4. Conservation mass, momentum, PHY 231, General University Physics (first part of two-semester survey of energy, and charge classical physics; consequences of the principles of mechanics are developed conceptually, analytically, and quantitatively) PHY 241, General University Physics Laboratory (laboratory course offering experiments in mechanics and heat, framed in a small group environment that requires coordination and team work in the development of a well written lab report) 5. Physical properties of matter PHY 232, General University Physics (a general course covering electricity, magnetism, electromagnetic waves and physical optics) PHY 242, General University Physics Laboratory (laboratory course offering experiments in electricity, magnetism, and light, framed in a small group environment that requires coordination and team work in the development of a well written lab report) 6. Kinetic-molecular motion and PHY 361, Modern Physics (elements of symmetry and special relative, atomic models notions of time and special relativity, Doppler effect and expanding universe, space-time and twin paradox, unification of electricity and magnetism, energy and momentum in special relativity, what is mass?, blackbody radiation, photoelectric effect, nature of light, Compton effect and pair production, De Broglie waves, interference of traveling matter waves, nature of standing matter waves, uncertainty principle, atoms scattering in the classical atom, atom light and thee quantum atom, atomic spectra, invention of lasers, wave functions and wave equations, Schroedinger’s equation, quantum operators, eigenvalues and eigenvectors, harmonic oscillator, electron probability density, electron spin, nuclear properties, liquid drop model, nuclear shell model, classification of forces and particles, symmetry principles and conservation laws, standard model and physics beyond) 41 7. Radioactivity, nuclear reactors, fission, and fusion 8. Wave theory, sound, light, the electromagnetic spectrum and optics 9. Electricity and magnetism 10. Fundamental processes of investigating in physics PHY 361, Modern Physics (elements of symmetry and special relative, notions of time and special relativity, Doppler effect and expanding universe, space-time and twin paradox, unification of electricity and magnetism, energy and momentum in special relativity, what is mass?, blackbody radiation, photoelectric effect, nature of light, Compton effect and pair production, De Broglie waves, interference of traveling matter waves, nature of standing matter waves, uncertainty principle, atoms scattering in the classical atom, atom light and thee quantum atom, atomic spectra, invention of lasers, wave functions and wave equations, Schroedinger’s equation, quantum operators, eigenvalues and eigenvectors, harmonic oscillator, electron probability density, electron spin, nuclear properties, liquid drop model, nuclear shell model, classification of forces and particles, symmetry principles and conservation laws, standard model and physics beyond) PHY 361, Modern Physics (elements of symmetry and special relative, notions of time and special relativity, Doppler effect and expanding universe, space-time and twin paradox, unification of electricity and magnetism, energy and momentum in special relativity, what is mass?, blackbody radiation, photoelectric effect, nature of light, Compton effect and pair production, De Broglie waves, interference of traveling matter waves, nature of standing matter waves, uncertainty principle, atoms scattering in the classical atom, atom light and thee quantum atom, atomic spectra, invention of lasers, wave functions and wave equations, Schroedinger’s equation, quantum operators, eigenvalues and eigenvectors, harmonic oscillator, electron probability density, electron spin, nuclear properties, liquid drop model, nuclear shell model, classification of forces and particles, symmetry principles and conservation laws, standard model and physics beyond) PHY 231, General University Physics (first part of two-semester survey of classical physics; consequences of the principles of mechanics are developed conceptually, analytically, and quantitatively) PHY 241, General University Physics Laboratory (laboratory course offering experiments in mechanics and heat, framed in a small group environment that requires coordination and team work in the development of a well written lab report) PHY 232, General University Physics (a general course covering electricity, magnetism, electromagnetic waves and physical optics) PHY 242, General University Physics Laboratory (laboratory course offering experiments in electricity, magnetism, and light, framed in a small group environment that requires coordination and team work in the development of a well written lab report) PHY 241, General University Physics Laboratory (laboratory course offering experiments in mechanics and heat, framed in a small group environment that requires coordination and team work in the development of a well written lab report) PHY 242, General University Physics Laboratory (laboratory course offering experiments in electricity, magnetism, and light, framed in a small group environment that requires coordination and team work in the development of a well written lab report) 11. Applications of physics in environmental quality and to personal and community health Table II: Physics – Advanced Competencies B. Advanced Competencies – In addition to the core competencies, teachers of physics as a primary field Required Courses should be prepared to effectively lead students to understand: 12. Thermodynamics and relationships PHY 361, Modern Physics (elements of symmetry and special relative, 42 between energy and matter 13. Nuclear physics including matterenergy duality and reactivity 14. Angular rotation and momentum, centripetal forces, and vector analysis 15. Quantum mechanics, space-time relationships, and special relativity 16. Models of nuclear and subatomic structures and behavior notions of time and special relativity, Doppler effect and expanding universe, space-time and twin paradox, unification of electricity and magnetism, energy and momentum in special relativity, what is mass?, blackbody radiation, photoelectric effect, nature of light, Compton effect and pair production, De Broglie waves, interference of traveling matter waves, nature of standing matter waves, uncertainty principle, atoms scattering in the classical atom, atom light and thee quantum atom, atomic spectra, invention of lasers, wave functions and wave equations, Schroedinger’s equation, quantum operators, eigenvalues and eigenvectors, harmonic oscillator, electron probability density, electron spin, nuclear properties, liquid drop model, nuclear shell model, classification of forces and particles, symmetry principles and conservation laws, standard model and physics beyond) PHY 361, Modern Physics (elements of symmetry and special relative, notions of time and special relativity, Doppler effect and expanding universe, space-time and twin paradox, unification of electricity and magnetism, energy and momentum in special relativity, what is mass?, blackbody radiation, photoelectric effect, nature of light, Compton effect and pair production, De Broglie waves, interference of traveling matter waves, nature of standing matter waves, uncertainty principle, atoms scattering in the classical atom, atom light and thee quantum atom, atomic spectra, invention of lasers, wave functions and wave equations, Schroedinger’s equation, quantum operators, eigenvalues and eigenvectors, harmonic oscillator, electron probability density, electron spin, nuclear properties, liquid drop model, nuclear shell model, classification of forces and particles, symmetry principles and conservation laws, standard model and physics beyond) PHY 231, General University Physics (first part of two-semester survey of classical physics; consequences of the principles of mechanics are developed conceptually, analytically, and quantitatively) PHY 241, General University Physics Laboratory (laboratory course offering experiments in mechanics and heat, framed in a small group environment that requires coordination and team work in the development of a well written lab report) PHY 361, Modern Physics (elements of symmetry and special relative, notions of time and special relativity, Doppler effect and expanding universe, space-time and twin paradox, unification of electricity and magnetism, energy and momentum in special relativity, what is mass?, blackbody radiation, photoelectric effect, nature of light, Compton effect and pair production, De Broglie waves, interference of traveling matter waves, nature of standing matter waves, uncertainty principle, atoms scattering in the classical atom, atom light and thee quantum atom, atomic spectra, invention of lasers, wave functions and wave equations, Schroedinger’s equation, quantum operators, eigenvalues and eigenvectors, harmonic oscillator, electron probability density, electron spin, nuclear properties, liquid drop model, nuclear shell model, classification of forces and particles, symmetry principles and conservation laws, standard model and physics beyond) PHY 361, Modern Physics (elements of symmetry and special relative, notions of time and special relativity, Doppler effect and expanding universe, space-time and twin paradox, unification of electricity and magnetism, energy and momentum in special relativity, what is mass?, blackbody radiation, photoelectric effect, nature of light, Compton effect and pair production, De Broglie waves, interference of traveling matter waves, nature of standing matter waves, uncertainty principle, atoms scattering in the classical atom, atom light and thee quantum atom, atomic spectra, invention of lasers, wave functions and wave equations, Schroedinger’s equation, quantum operators, eigenvalues and eigenvectors, harmonic oscillator, electron probability density, electron spin, nuclear properties, liquid drop model, nuclear shell model, classification of forces and particles, symmetry principles and conservation laws, standard model and physics beyond) 43 17. Light behavior, including waveparticle duality and models 18. Electrical phenomena including electric fields, vector analysis, energy, potential, capacitance, and inductance 19. Issues related to physics such as disposal of nuclear waste, light pollution, shielding communication systems and weapons development 20. Historical development and cosmological perspectives in physics including contributions of significant figures and underrepresented groups, and evolution of theories in physics 21. How to design, conduct, and report research in physics 22. Applications of physics and engineering in society, business, industry, and health fields PHY 361, Modern Physics (elements of symmetry and special relative, notions of time and special relativity, Doppler effect and expanding universe, space-time and twin paradox, unification of electricity and magnetism, energy and momentum in special relativity, what is mass?, blackbody radiation, photoelectric effect, nature of light, Compton effect and pair production, De Broglie waves, interference of traveling matter waves, nature of standing matter waves, uncertainty principle, atoms scattering in the classical atom, atom light and thee quantum atom, atomic spectra, invention of lasers, wave functions and wave equations, Schroedinger’s equation, quantum operators, eigenvalues and eigenvectors, harmonic oscillator, electron probability density, electron spin, nuclear properties, liquid drop model, nuclear shell model, classification of forces and particles, symmetry principles and conservation laws, standard model and physics beyond) PHY 232, General University Physics (a general course covering electricity, magnetism, electromagnetic waves and physical optics) PHY 242, General University Physics Laboratory (laboratory course offering experiments in electricity, magnetism, and light, framed in a small group environment that requires coordination and team work in the development of a well written lab report) AST 191, The Solar System [Recommended] (emphasizing nature, origin, and evolution of planets, satellites, and other objects in the solar system; topics also include historical astronomy, the naked eye phenomenon of the sky and modern solar system discoveries made by spacecraft) PHY 241, General University Physics Laboratory (laboratory course offering experiments in mechanics and heat, framed in a small group environment that requires coordination and team work in the development of a well written lab report) PHY 242, General University Physics Laboratory (laboratory course offering experiments in electricity, magnetism, and light, framed in a small group environment that requires coordination and team work in the development of a well written lab report) PHY 361, Modern Physics (elements of symmetry and special relative, notions of time and special relativity, Doppler effect and expanding universe, space-time and twin paradox, unification of electricity and magnetism, energy and momentum in special relativity, what is mass?, blackbody radiation, photoelectric effect, nature of light, Compton effect and pair production, De Broglie waves, interference of traveling matter waves, nature of standing matter waves, uncertainty principle, atoms scattering in the classical atom, atom light and thee quantum atom, atomic spectra, invention of lasers, wave functions and wave equations, Schroedinger’s equation, quantum operators, eigenvalues and eigenvectors, harmonic oscillator, electron probability density, electron spin, nuclear properties, liquid drop model, nuclear shell model, classification of forces and particles, symmetry principles and conservation laws, standard model and physics beyond) Table III: Physics – Supporting Competencies C. Supporting Competencies – All teachers of physics should be prepared to effectively apply concepts from other sciences and Required Courses mathematics to the teaching of physics including concepts of: 23. Biology, including organization of BIO 150, Principles of Biology I (develop appreciation of biological 44 life, bioenergetics, biomechanics, and cycles of matter 24. Chemistry, including organization of matter and energy, electrochemistry, thermodynamics, and bonding 25. Earth sciences or astronomy related to structure of the universe, energy, and interactions of matter 26. Mathematical and statistical concepts and skills including statistics and the use of differential equations and calculus principles necessary to explore life at the cellular and molecular level; similarities and differences in structure and function of simple and complex cells covered along with theories on the origin and evolution of biological systems) BIO 151, Principles of Biology Laboratory I (introductory laboratory in which biological systems are investigated at the cellular and molecular levels) CHE 105, General College Chemistry I (basic atomic structure, bonding, reactions, periodicity, thermodynamics, intermolecular forces, quantum theory, hybridization, mass relationships in chemical reactions, gases) CHE 107, General College Chemistry II (kinetics, acid-base, thermodynamics, electrochemistry, molecular geometry, hybridization, intermolecular forces, physical properties of solutions, equilibrium) CHE 115, General Chemistry Laboratory (separations, synthesis, measurements, mathematical applications, basic laboratory skills, safety) GLY 220, Principles of Physical Geology (integrated course in physical geology covering the physical, chemical, and biological processes that combine to produce geological processes; attention is focused on plate tectonics, earth surface processes, and properties and formation of earth materials; laboratory experiences emphasize identification and interpretation of geological materials and maps) AST 191, The Solar System [Recommended] (emphasizing nature, origin, and evolution of planets, satellites, and other objects in the solar system; topics also include historical astronomy, the naked eye phenomenon of the sky and modern solar system discoveries made by spacecraft) MA 113, Calculus I (course in one variable calculus, including topics from analytic geometry; derivatives and integrals of elementary functions – including trigonometric functions – with applications) MA 114 Calculus II (a continuation of MA 113, primarily stressing techniques of integration) PHY 231, General University Physics (first part of two-semester survey of classical physics; consequences of the principles of mechanics are developed conceptually, analytically, and quantitatively) PHY 232, General University Physics (a general course covering electricity, magnetism, electromagnetic waves and physical optics) PHY 361, Modern Physics (elements of symmetry and special relative, notions of time and special relativity, Doppler effect and expanding universe, space-time and twin paradox, unification of electricity and magnetism, energy and momentum in special relativity, what is mass?, blackbody radiation, photoelectric effect, nature of light, Compton effect and pair production, De Broglie waves, interference of traveling matter waves, nature of standing matter waves, uncertainty principle, atoms scattering in the classical atom, atom light and thee quantum atom, atomic spectra, invention of lasers, wave functions and wave equations, Schroedinger’s equation, quantum operators, eigenvalues and eigenvectors, harmonic oscillator, electron probability density, electron spin, nuclear properties, liquid drop model, nuclear shell model, classification of forces and particles, symmetry principles and conservation laws, standard model and physics beyond) F. Integration of Code of Ethics into Science Education Program Candidates are required to complete and submit a Code of Ethics Form with applications for admission to the program. The document must be signed by the applicant and the form includes an attachment describing professional/ethical expectations for teachers. Candidates replying “yes” to any possible ethics violation are asked to make an appointment with the Director of Academic Services and Teacher Certification. The Director works with the candidate in gathering additional information regarding documenting the case and the disposition of the case. At that point, the Director discusses 45 the case with representatives of the Education Professional Standards Board and a decision is reached regarding any potential problems with future certification. These activities occur prior to admission to the program. Following admissions, references are made in MIC Program Orientation and cohort sessions prior to and during fall field placement regarding expectations of professional conduct. In their cohort, candidates read and discuss The Ethics of Teaching by Strike and Soltis published in 2004 by Teachers College Press. This resource is used to help candidates make decisions regarding ethical decisions and activities of the classroom teacher. Candidates are expected to demonstrate appropriate, professional behavior in discussing students and/or student work within, and outside, the classroom. The science teaching methods course includes information and issues related to proving healthy and safe environments in the science classroom (laboratory). Further discussions are held regarding the ethics of accurate reporting of scientific data and the importance of basing interpretations upon the question asked and data collected. G. Integration of KERA Initiatives into Science Education Program Candidates have multiple opportunities to become aware of, and use, KDE (Kentucky Department of Education) documents (e.g., KERA initiatives) related to student outcomes and expectations. Candidates from the early stages of lesson preparation are required to align lessons with these documents. The same is true in their preparation of lessons conducted in classrooms. A lesson plan is incomplete without this alignment. References to KERA initiatives are included in both cohort and special methods courses. As a first assignment in science teaching methods, candidates are asked to download these documents from the KDE website (see EDC 634 syllabus). While it seems awkward to compare university content courses with outcomes expected of high school students, a content alignment follows. This alignment demonstrates that topics covered in university content courses are supportive of concepts and skills expected of high school science students. Alignment with KERA Initiatives: In this document, the Kentucky Academic Expectations for Science, the Program of Studies for High School Science, and the Core Content for High School Science are compared to the course requirements for certification in secondary science at the University of Kentucky. A section is defined by one of the six Academic Expectations for Science. In each section, the associated Program of Studies and Core Content statements are listed and grouped in boxes. Under a given box, a brief paragraph describes the relationship between the statements and the course requirements for the Science Education Program. Section 1 2.1 Students understand scientific ways of thinking and working and use those methods to solve real-life problems S-HS-SI-1 (program of studies) Students will identify and refine questions and identify scientific concepts to guide the design of scientific investigations. S-HS-SI-1 (program of studies) Students will identify and refine questions and identify scientific concepts to guide the design of scientific investigations. S-HS-SI-2 (program of studies) Students will design and conduct different kinds of scientific investigations for a wide variety of reasons. S-HS-SI-3 (program of studies) Students will use equipment (e.g., microscopes, lasers), tools (e.g., beakers), techniques (e.g., microscope skills), technology (e.g., computers), and mathematics to improve scientific investigations and communications. S-HS-SI-4 (program of studies) Students will use evidence, logic, and scientific knowledge to develop and revise scientific explanations and models. S-HS-SI-5 (program of studies) Students will communicate designs, procedures, and results of scientific investigations. SC-HS-1.1 8 Students will explain the importance of chemical reactions in a real-world context; justify conclusions using evidence/data from chemical reactions. (core content) SC-HS-4.6.7 Students will explain real world applications of energy using information/data; evaluate explanations of mechanical systems using current scientific knowledge about energy. (core content) S-HS-SI-6 (program of studies) 46 Students will review and analyze scientific investigations and explanations of others. S-HS-AC-7 (program of studies) Students will use science to investigate natural hazards and human-induced hazards S-HS-AC-8 (program of studies) Students will analyze how science and technology are necessary but not sufficient for solving local, national, and global issues. S-HS-AC-9 (program of studies) Students will analyze the role science plays in everyday life and compare different careers in science. S-HS-AC-10 (program of studies) Students will recognize that scientific knowledge comes from empirical standards, logical arguments, skepticism, and is subject to change as new evidence becomes available. S-HS-AC-11 (program of studies) Students will investigate advances in science and technology that have important and long- lasting effects on science and society (e.g., Newtonian mechanics, plate tectonics, germ theory, medical and health technology). SC-HS-4.7.2 Students will evaluate proposed solutions from multiple perspectives to environmental problems caused by human interaction; justify positions using evidence/data. (core content) SC-HS-4.7.5 Students will predict the consequences of changes in resources to a population; select or defend solutions to real-world problems of population control. (core content) SC-HS-2.3.8 Students will compare the limitations/benefits of various techniques ( radioactive dating, observing rock sequences, and comparing fossils) for estimating geological time; justify deductions about age of geologic features. (core content) The following courses are required and taken by all secondary science education majors: Chemistry 115 (Introduction to General Chemistry Laboratory), Geology 220 (Principles of Physical Geology), Biology 151 (Introduction to Biology Laboratory I), and Education-Curriculum and Instruction 634 (Science Pedagogy in the Secondary School). In all of these courses, candidates use discipline specific laboratory equipment to conduct investigations. Candidates are expected to communicate the results of investigations via laboratory reports. Candidates justify their conclusions using data collected from the laboratory investigations and theoretical lectures. In EDC 634, candidates design laboratory experiments, conduct investigations and submit laboratory reports. Lab reports are individual, but the laboratory work is completed in cooperative groups. Candidates must identify investigative question(s) prior to designing the experiment. Candidates following the sequence of courses for certification in biology obtain advanced experience and skills in Chemistry 231 (Organic Chemistry Laboratory I), Chemistry 233 (Organic Chemistry Laboratory II) and Biology 153 (Principles of Biology Laboratory II). Candidates pursuing certification in chemistry obtain advanced experience and skills in Chemistry 231 (Organic Chemistry Laboratory I) and Chemistry 233 (Organic Chemistry Laboratory II). Those candidates seeking certification in earth sciences gain additional experiences in Geology 230 (Fundamentals of Geology I), and Geology 235 (Fundamentals of Geology II). A selection between Geology 401G (Invertebrate Paleobiology) or Geology 360 (Mineralogy) provides additional specialized laboratory and field experiences. Section 2 2.2 Students identify, analyze, and use patterns such as cycles and trends to understand past and present events and predict possible future events. S-HS-PS-1 (Program of studies) Students will analyze atomic structure and electric forces. SC-HS-1.1.1 Students will classify or make generalizations about elements from data of observed patterns in atomic structure and/or position on the periodic table. (core content) SC-HS-1.1.2 Students will understand that the atom’s nucleus is composed of protons and neutrons that 47 are much more massive than electrons. When an element has atoms that differ in the number of neutrons, these atoms are called different isotopes of the element. (core content) SC-HS-4.6.6 Students will understand that heat is the manifestation of the random motion and vibrations of atoms. (core content) All students seeking certification in secondary science are required to take Chemistry 105 (General College Chemistry I), Chemistry 107 (General College Chemistry II) and Chemistry 115 (General Chemistry Laboratory). Atomic structure and periodicity are explicitly addressed in these courses via homework, class tests and the final exam. S-HS-PS-2 (program of studies) Students will examine nuclear structure, nuclear forces, and nuclear reactions (e.g., fission, fusion, radioactivity). Secondary science education majors seeking certification take Chemistry 105 (Introduction to Chemistry I) and Chemistry 107 (Introduction to Chemistry II). In this series of courses, nuclear structure, nuclear reactions and nuclear forces are taught and tested. Candidates seeking certification in physics take Physics 361 (Principles of Modern Physics). Candidates study the nucleus both its structure and function in greater depth. S-HS-PS-4 (program of studies) Students will investigate how the structure of matter (e.g., constituent atoms, distances and angles between atoms) relates to physical properties of matter. SC-HS-1.1.6 Students will identify variables that affect reaction rates; predict effects of changes in variables (concentration, temperature, properties of reactants, surface area, and catalysts) based on evidence/data from chemical reactions. (core content) Candidates following the requirements for certification in secondary are required to take Chemistry 105 (General College Chemistry I) and Chemistry 107 (General College Chemistry II). Structure of matter and reactions behavior is included within the course content. Biology and chemistry certification candidates are required to take Chemistry 230 (Organic Chemistry I) and Chemistry 232 (Organic Chemistry II). Both of these courses study structure of matter, reaction rates and physical properties of matter. SC-HS-1.1.3 Students will understand that solids, liquids, and gases differ in the distances between molecules or atoms and therefore the energy that binds them together. In solids, the structure is nearly rigid; in liquids, molecules or atoms move around each other but do not move apart; and in gases, molecules or atoms move almost independently of each other and are relatively far apart. (core content) S-HS-PS-5 (program of studies) Students will investigate chemical reactions and the release or consumption of energy. For certification, all secondary science education majors must pass Chemistry 105 (General College Chemistry I) and Chemistry 107 (General College Chemistry II). The structure and energy relationships of matter are investigated. Chemistry certification requires candidates to enroll in Chemistry 440G (Introductory Physical Chemistry). This course provides these candidates a greater depth of knowledge for these content statements. S-HS-PS-7 (program of studies) Students will investigate factors (e.g., temperature, catalysts) affecting reaction rates. All candidates seeking secondary science certification are required to pass Chemistry 115 (General Chemistry Laboratory). In this course, candidates investigate the phenomenon in laboratory experiments. 48 Knowledge and experience with these concepts are increased for chemistry and biology certification seekers by taking Chemistry 231 (Organic Chemistry Laboratory I) and Chemistry 232 (Organic Chemistry Laboratory II). These two courses focus upon reaction mechanisms and the factors affecting their rates and yields. S-HS-PS-9 (program of studies) Students will investigate gravitational and electromagnetic forces. SC-HS-4.6.3 Students will understand that electromagnetic waves, including radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, x-rays, and gamma rays, result when a charged object is accelerated. (core content) SC-HS-4.6.2 Students will predict wave behavior and energy transfer; apply knowledge of waves to real life phenomena/investigations. (core content) All students at the University of Kentucky are required to take a physics sequence. They select from Physics 211/213 (General Physics Series) or Physics 231/232 (General University Physics) with Physics 241/242 (General University Physics Laboratory). In these courses, the basics of gravitational and electromagnetic forces are discussed and evaluated. Candidates seeking certification in physics must take Physics 361 (Principles of Modern Physics). Physics students will obtain a greater depth of knowledge for these concepts. S-HS-PS-11 (program of studies) Students will distinguish between types of energy (e.g., kinetic energy, potential energy, energy fields). S-HS-PS-14 (program of studies) Students will investigate electrical energy and conductivity through matter. SC-HS-4.6.1 Students will explain the relationships and connections between matter, energy, living systems, and the physical environment; give examples of conservation of matter and energy. (core content) All students at the University of Kentucky are required to take physics sequence. They select from Physics 211/213 (General Physics Series) or Physics 231/232 (General University Physics) with Physics 241/242 (General University Physics Laboratory). These series of courses address these concepts related to energy and matter. S-HS-ESS-1 (program of studies) Students will examine internal and external sources of energy. S-HS-ESS-3 (program of studies) Students will examine how external sources of energy produce winds and ocean currents. S-HS-ESS-5 (program of studies) Students will recognize that the Earth contains a fixed amount of each stable chemical atom or element. Principles of Physical Geology (GEO 220) is required for all candidates in secondary science education. Candidates study the composition of the earth by atoms/elements, winds, and energy sources. S-HS-ESS-4 (program of studies) Students will examine how external sources of energy determine global climate. SC-HS-4.6.9 Students will explain the cause and effect relationship between global climate and weather patterns and energy transfer (cloud cover, location of mountain ranges, oceans); predict the consequences of changes to the global climate and weather patterns (core content) University of Kentucky Science Education majors are all expected to take and pass Geology 220 (Principles of Physical Geology). These concepts are addressed in lecture and in associated laboratory experiments. 49 Candidates pursuing earth science certification gain advanced understanding of these concepts in either Geography 130 (Earth’s Physical Environment) or Geography 251 (Weather and Climate). S-HS-ESS-6 (program of studies) Students will analyze Earth’s chemical reservoirs and recognize that each element can exist in several reservoirs (e.g., carbon in carbon dioxide reservoirs and carbonate reservoirs). Geology 220 (Principles of Physical Geology) is a required course for all secondary science education majors. This course investigates the various forms of elements in our environment. Earth science certification candidates must enroll in Geology 230 (Fundamentals of Geology I) and Geology 235 (Fundamentals of Geology II). These courses increase candidates’ knowledge of chemical reservoirs and the forms of the element. S-HS-ESS-8 (program of studies) Students will describe the formation of the solar system. SC-HS-2.3.7 Students will understand that the Sun, Earth, and the rest of the solar system formed approximately 4.6 billion years ago from a nebular cloud of dust and gas. (core content) Earth science certification majors are required to take Astronomy 191 (The Solar System). This course is also recommended for other science education majors. The class discusses the formation of the solar system and its historical development. S-HS-LS-6 (program of studies) Students will examine diversity of organisms and biological classification. SC-HS-3.4.7 Students will classify organisms into groups based on similarities; infer relationships based on internal and external structures and chemical processes. (core content) University of Kentucky secondary science education majors must take Biology 150 (Principles of Biology I) and Biology 151 (Principles of Biology Laboratory I). These two courses provide foundational knowledge of classification systems and body structure. Candidates seeking certification in biology deepen their understanding in Biology 152 (Principles of Biology II), Biology 153 (Principles of Biology Laboratory II), Biology 351 (Plant Kingdom) or Biology 430G (Plant Physiology) and Biology 350 (Animal Physiology). S-HS-LS-7 (program of studies) Students will investigate the cycle of atoms (e.g., carbon) and molecules (e.g., nitrogen, carbon dioxide, oxygen) within the biosphere. SC-HS-4.6.4 Students will describe the components and reservoirs involved in biogeochemical cycles ( water, nitrogen, carbon dioxide, and oxygen); explain the movement of matter and energy in biogeochemical cycles and related phenomena. (core content) SC-HS-4.6.10 Students will identify the components and mechanisms of energy stored and released from food molecules (photosynthesis and respiration); apply information to real-world situations. (core content) Secondary science education majors must include Biology 150 (Principles of Biology I) and Biology 151 (Principles of Biology Laboratory I) in their course of study. These two courses provide foundational knowledge of classification systems and body structure. SC-HS-4.6.5 Students will describe and explain the role of carbon-containing molecules and chemical reactions in energy transfer in living systems. (core content) 50 Chemistry certification majors enroll in Biochemistry 401G (Fundamentals of Biochemistry) to learn about chemistry in living systems. Biology certification majors must take Biology 325 (Introductory Ecology) to increase their knowledge of energy transfer in living systems. SC-HS-4.7.4 Students will understand that evidence for one-celled forms of life, the bacteria, extends back more than 3.5 billion years. The changes in life over time caused dramatic changes in the composition of the Earth’s atmosphere, which did not originally contain oxygen. (core content) All secondary science education majors are required to pass Biology 150 (Principles of Biology I) and Biology 151 (Principles of Biology Laboratory I). In addition, candidates must take Geology 220 (Principles of Physical Geology). Both of these courses strengthen candidates’ knowledge of the interrelationship between the earth’s formation and the development of life on earth. Section 3 2.3 Students identify and analyze systems and the ways their components work together or affect each other. S-HS-PS-3 (program of studies) Students will investigate how the structure of matter (e.g., outer electrons, type of bond) relates to chemical properties of matter. SC-HS-1.1.5 Students will explain the role of intermolecular or intramolecular interactions on the physical properties (solubility, density, polarity, boiling/melting points) of compounds. (core content) S-HS-PS-6 (program of studies) Students will examine the transfer of electrons or hydrogen ions between reacting ions, molecules, or atoms. SC-HS-1.1.7 Students will construct diagrams to illustrate ionic or covalent bonding; predict compound formation and bond type as either ionic or covalent (polar, nonpolar). (core content) All candidates seeking certification in secondary science education are required to pass Chemistry 105 (Introduction to Chemistry I), Chemistry 107 (Introduction to Chemistry II), and Chemistry 117 (Introduction to Chemistry Laboratory). These three courses address the structure of matter and its relationship to chemical properties, intermolecular forces, and reaction mechanisms. Candidates seeking certification in chemistry and biology deepen their knowledge of these concepts in Chemistry 230 (Organic Chemistry I) and Chemistry 232 (Organic Chemistry II). SC-HS-1.1.4 Students will understand that in conducting materials, electrons flow easily; whereas, in insulating materials, they can hardly flow at all. Semiconducting materials have intermediate behavior. At low temperatures, some materials become superconductors and offer no resistance to the flow of electrons. (core content) Secondary science education majors will study the concepts in SC-HS-1.1.4 from taking and passing Chemistry 105 (Introduction to Chemistry I) and taking one of the following physics series: Physics 211/213 (General Physics) or Physics 231/231 (General University Physics). S-HS-PS-8 (program of studies) Students will investigate forces and the effects of forces on the motion of objects. SC-HS-2.3.1 Students will explain phenomena (falling objects, planetary motion, satellite motion) related to gravity; describe the factors that affect gravitational force (core content) 51 To complete a secondary science education major, candidates must take one of the following physics series: Physics 211/213 (General Physics) or Physics 231/231 (General University Physics). In either series, the topics of force and motion are investigated. For completion of a science education major, candidates will enroll in and pass one of the following physics series: Physics 211/213 (General Physics) or Physics 231/231 (General University Physics). These series introduce candidates to the concepts of electromagnetism and nuclear behavior. Candidates earning a physics education degree will receive advanced competency of these concepts in Physics 361 (Principles of Modern Physics). S-HS-ESS-7 (program of studies) Students will investigate how Earth’s internal and external sources of energy drive geochemical cycles (e.g., carbon moving from carbon dioxide reservoirs to carbonate reservoirs). University of Kentucky secondary education majors are required to take Geology 220 (Principles of Physical Geology). This course investigates geochemical cycles. SC-HS-2.3.3 Students will explain the origin of the heavy elements in planetary objects (planets, stars). (core content) Candidates seeking certification in earth science are required to take Astronomy 191 (The Solar System), In addition, candidates are highly recommended to take Astronomy 192 (The Universe). Astronomy 191 and Astronomy 192 both discuss the origin of elements and their use to form planetary objects. S-HS-ESS-12 (program of studies) Students will describe the formation of the stars. SC-HS-2.3.4 Students will understand that stars have life cycles of birth through death that are analogous to those of living organisms. During their lifetimes, stars generate energy from nuclear fusion reactions that create successively heavier chemical elements. (core content) S-HS-ESS-13 (program of studies) Students will examine stars (e.g., energy production, formation of elements). Science education majors are highly recommended to take Astronomy 192 (The Universe). This course discusses the formation of the universe from the big bang to the formation of stars. S-HS-LS-1 (program of studies) Students will investigate cell structures, their functions (e.g., chemical reactions), and how DNA guides their functions. S-HS-LS-2 (program of studies) Students will investigate cell regulation, differentiation, and how the process of photosynthesis provides a vital connection between the Sun and energy needs of living systems. SC-HS-3.4.2 Students will understand that most cell functions involve chemical reactions. Food molecules taken into cells react to provide the chemical constituents needed to synthesize other molecules. Both breakdown and synthesis are made possible by a large set of protein catalysts, called enzymes. The breakdown of some of the food molecules enables the cell to store energy in specific chemicals that are used to carry out the many functions of the cell. (core content) SC-HS-3.4.3 Students will describe cell regulation (enzyme function, diffusion, osmosis, homeostasis); predict consequences of internal/external environmental change on cell function/regulation. (core content) S-HS-LS-3 (program of studies) Students will investigate how DNA carries instructions for specifying characteristics of organisms. SC-HS-3.4.1 Students will explain the role of DNA in protein synthesis. (core content) S-HS-LS-4 (program of studies) Students will investigate encoding and replication of genetic information. 52 SC-HS-3.4.5 Students will explain the relationship between sexual reproduction (meiosis) and the transmission of genetic information; draw conclusions/make predictions based on hereditary evidence/data (pedigrees, punnet squares). (core content) SC-HS-3.4.6 Students will understand that in all organisms and viruses, the instructions for specifying the characteristics are carried in nucleic acids. The chemical and structural properties of nucleic acids determine how the genetic information that underlies heredity is both encoded in genes and replicated. (core content) All secondary science education majors are required to pass Biology 150 (Introduction to Biology I) and Biology 151 (Introduction to Biology Laboratory I). This course provides candidates with a foundation in cell structure, cell function, cell regulation and cell respiration. Candidates seeking a biology certification must take either Biology 304 (Principles of Genetics) or Agricultural Biotechnology 360 (Genetics). The basic foundation formed in Biology 150 and 151 are enhanced in these courses. Candidates seeking chemistry certification must take Biochemistry 401G (Fundamentals of Biochemistry) which increases their knowledge of these genetic concepts. SC-HS-3.4.4 Students will understand that plant cells contain chloroplasts, the site of photosynthesis. Plants and many microorganisms (e.g., Euglena) use solar energy to combine molecules of carbon dioxide and water into complex, energy-rich organic compounds and release oxygen to the environment. This process of photosynthesis provides a vital link between the Sun and energy needs of living systems. (core content) S-HS-LS-11 (program of studies) Students will recognize that living systems require continuous input of energy. S-HS-LS-12 (program of studies) Students will investigate photosynthesis, cellular respiration, and the energy relationships among them. All secondary science education majors are required to take and pass Biology 150 (Introduction to Biology I) and Biology 151 (Introduction to Biology Laboratory I). In this course students will develop the concepts of photosynthesis. Biology certification requires candidates to pass either Biology 351(The Plant Kingdom) or Biology 430G (Plant Physiology). Either of these courses increases knowledge of the photosynthesis process in plants. S-HS-LS-13 (program of studies) Students will analyze the flow of matter and energy through and between living systems and environments. All candidates are required to pass Biology 150 (Introduction to Biology I), Biology 151 (Introduction to Biology Laboratory 1) and Geology 220 (Principles of Physical Geology). Each of these courses develops the concept of the flow of matter between living systems and the environment from two perspectives. Candidates seeking biology certification will receive advanced study of these concepts by taking Biology 325 (Ecology). SC-HS-3.4.8 Students will understand that multicellular animals have nervous systems that generate behavior. Nerve cells communicate with each other by secreting specific molecules. Specialized cells in sense organs detect light, sound, and specific chemicals enabling animals to monitor what is going on in the world around them.(core content) All secondary education majors must take Biology 151(Introduction to Biology I) and Biology 151 (Introduction to Biology Laboratory I). This course develops a fundamental concept of multicellular animals. Biology certification majors will select from a variety of courses to develop advanced understanding of these concepts. 53 S-HS-AC-2 (program of studies) Students will examine the interaction between science and technology. Candidates must take education course EDC 634 (Science Pedagogy in the Secondary School). The difference between science and technology is explored as well as how the two areas work together enhancing our understanding of the world. S-HS-AC-6 (program of studies) Students will investigate how science can be used to solve environmental quality problems (e.g., overconsumption, food distribution). Candidates seeking science certification are required to pass Chemistry 105 (Introduction to Chemistry I) and Chemistry 107 (Introduction to Chemistry II). Candidates must take Geology 220 (Principles of Physical Geology). These three courses include environmental aspects exploring how science improves our environment and how it improves our lives. Section 4 2.4 Students use the concept of scale and scientific models to explain the organization and functioning of living and nonliving things and predict other characteristics that might be observed. S-HS-ESS-2 (program of studies) Students will examine how internal sources of energy propel crustal plates across the face of the globe. Geology 220 (Principles of Physical Geology) is required for all secondary science education majors. This course investigates plate tectonics and the possible driving forces for tectonic plates. S-HS-ESS-11 (program of studies) Students will describe theories of the formation of the universe (e.g., big bang theory). SC-HS-2.3.2 Students will describe the current scientific theory of the formation of the universe (Big Bang) and its evidence; explain the role of gravity in the formation of the universe and it’s components. (core content) Earth science majors are highly recommended to take Astronomy 192 (The Universe). Candidates in this course study the formation of the universe. S-HS-LS-14 (program of studies) Students will investigate behavioral responses to internal changes and external stimuli. S-HS-LS-15 (program of studies) Students will analyze how patterns of behavior ensure reproductive success. All secondary education majors are required to take Biology 150 (Introduction to Biology I) and Biology 151 (Introduction to Biology Laboratory I). This course develops a foundation of behavioral responses and patterns in plants and animals. S-HS-AC-1 (program of studies) Students will apply scientific inquiry and conceptual understandings to solving problems of technological design (e.g., styrofoam cups, transistors, computer chips). University of Kentucky science education majors are required to pass EDC 634 (Science Pedagogy in the Secondary School). This course is designed to provide pre-service teachers experience developing and testing theories in an inquiry setting as well as how to use these strategies with high school students. SC-HS-1.2.1 Students will select or construct accurate and appropriate representations for motion (visual, graphical, and mathematical); defend conclusions/explanations about the motion of objects and real-life phenomena from evidence/data. (core content) 54 Secondary education majors are required to take a variety of classes to meet the educational requirement of this core content statement. Chemistry 115 (Introduction to Chemistry Laboratory), Biology 151 (Introduction to Biology Laboratory I), and Geology 220 (Principles of Physical Geology) are courses in which the candidates have a first hand experience with constructing graphs and interpreting these graphs. Candidates are also expected to take one of the following physics series Physics 211/213 (General Physics) or Physics 241/242 (General University Physics Laboratory). These series provide pre-service teachers with specific experiences with graphs of motion and interpretation. Section 5 2.5 Students understand that under certain conditions nature tends to remain the same or move toward a balance. S-HS-PS-10 (program of studies) Students will examine how energy is transferred (e.g., collisions, light waves) and recognize that the total energy of the universe is constant. Secondary science education majors are required to take one series of physics either Physics 211/213 (General Physics) or Physics 231/232 (General University Physics) with Physics 241/242 (General University Physics Laboratory). The concepts of light and waves are studied and evaluated during these course sequences. Candidates pursuing physics education receive advanced training of energy concepts in Physics 361 (Principles of Modern Physics). S-HS-LS-8 (program of studies) Students will analyze energy flow through ecosystems. S-HS-LS-9 (program of studies) Students will examine interrelationships and interdependencies of organisms in ecosystems and the factors that influence the interactions between organisms. S-HS-AC-4 (program of studies) Students will recognize how science influences human population growth. All secondary science education students must pass Biology 150 (Introduction to Biology I) and Biology 151 (Introduction to Biology Laboratory I). These two courses develop basic conceptual understanding in ecosystems and human population growth of ecosystems. Candidates seeking biology certification increase their knowledge of these concepts in Biology 325 (Ecology). Section 6 2.6 Students understand how living and nonliving things change over time and the factors that influence the changes. S-HS-PS-12 (program of studies) Students will examine how everything tends to become less organized and less orderly over time (e.g., heat moves from hotter to cooler objects). Candidates seeking secondary science education degrees are required to take Chemistry 105 (Introduction to Chemistry I), Chemistry 107 (Introduction to Chemistry II), and one series of physics either Physics 211/213 (General Physics) or Physics 231/232 (General University Physics). These courses discuss enthalpy from a chemistry and physics perspective. Physics and chemistry certification majors take courses that provide greater depth of knowledge in entropy. For chemistry students, one required course is Chemistry 440G (Introductory Physical Chemistry). S-HS-ESS-9 (program of studies) Students will investigate how to estimate geologic time (e.g., observing rock sequences, radioactive dating). S-HS-ESS-10 (program of studies) Students will examine and interpret ongoing changes of the Earth system (e.g., earthquakes, mountain building). SC-HS-2.3.9 Students will explain real-life phenomena caused by the convection of the Earth’s mantle; 55 predict the consequences of this motion on humans and other living things on the planet. (core content) SC-HS-2.3.10 Students will predict consequences of both rapid (volcanoes, earthquakes) and slow (mountain building, plate movement) earth processes from evidence/data and justify reasoning. (core content) SC-HS-4.6.8 Students will describe the connections between the functioning of the Earth system and its sources of energy (internal and external); predict the consequences of changes to any component of the Earth system. (core content) SC-HS-4.7.3 Students will predict the consequences of changes to any component (atmosphere, solid Earth, oceans, living things) of the Earth System; propose justifiable solutions to global problems. (core content) Candidates seeking a secondary science education degree take and must pass Geology 220 (Principles of Physical Geology). In Geology 220, the function and mechanics of volcanoes, geologic time and changes to the surface of the earth, tectonic movement, uniformitarianism and catastrophicism in earth processes, energy both internal and solar and prediction in changes to the surface of the earth are addressed in both lecture and laboratory activities. This course includes a limited amount of field work. Earth science education majors gain advanced knowledge from Geology 230 (Fundamentals of Geology I) and Geology 235 (Fundamentals of Geology II). S-HS-LS-5 (program of studies) Students will examine how species change over time. S-HS-LS-10 (program of studies) Students will explore how human activities alter ecosystems. To complete a degree in secondary science education, candidates must pass Biology 150 (Introduction to Biology I) and Biology 151 (Introduction to Biology Laboratory I). This course develops a fundamental understanding of evolution as well as how human activity affects ecosystems. SC-HS-3.5.1 Students will predict the impact on species of changes to 1) the potential for a species to increase its numbers, (2) the genetic variability of offspring due to mutation and recombination of genes, (3) a finite supply of the resources required for life, or (4) natural selection; propose solutions to real-world problems of endangered and extinct species. (core content) SC-HS-3.5.2 Students will predict the success of patterns of adaptive behaviors based on evidence/data; justify explanations of organism survival based on scientific understandings of behavior. (core content) SC-HS-4.7.1 Students will analyze relationships and interactions among organisms in ecosystems; predict the effects on other organisms of changes to one or more components of the ecosystem. (core content) Biology 150 (Introduction to Biology I) and Biology 151 (Introduction to Biology Laboratory I) are required for all secondary science education majors. These courses provide a basic fundamental conceptual understanding of the principles of evolution and ecology. Candidates seeking biology certification must take Biology 325 (Ecology) to develop in-depth understanding of these concepts. The biology department offers a specific evolution course that is not a required course for secondary biology majors, but has been taken by many past graduates. S-HS-AC-3 (program of studies) Students will explore the impact of scientific knowledge and discoveries on personal and community health. 56 Chemistry 105 (Introduction to Chemistry I) and Chemistry 107 (Introduction to Chemistry II) are required by all secondary education majors. In this course, candidates are exposed to readings that demonstrate the impact of scientific knowledge and discoveries on personal and community health. S-HS-AC-5 (program of studies) Students will use science to analyze the use of natural resources by an increasing human population. Biology majors take BIO 325, Ecology, and examine the interactions of living organisms, including humans, with the physical environment. H. Integration of EPSB Themes into the Science Education Program Diversity is addressed in all of the professional courses through various readings, discussions, and projects. Candidates are instructed in various instructional methods to help reach all students in their classrooms. One of their major projects this year is called the Campbellsville Project. In this project, candidates interact with freshmen students at Campbellsville High School via face-to-face and in an online environment. Candidates act as mentors to the freshmen students, concentrating on various communications skills rather than critiquing the work. Another way diversity is addressed in the program is through candidates’ field experiences. Candidates are placed in a different school with each observation/student teaching experience, giving them a minimum of three different observation/student teaching experience settings. Assessment is addressed in several of the professional courses (see table below). The focus on various types of assessment allows candidates to develop skills necessary to assess student learning effectively and efficiently. Literacy Education is addressed in the courses listed below. The focus on literacy in the core MIC courses is on developing a literate society. EDC 631 discusses more in-depth how to integrate research-based literacy strategies into the secondary mathematics classroom. Closing the Achievement Gap is addressed in several of the professional courses. First and foremost, research is presented indicating the achievement gap and reasons for the gap are discussed. Instructional strategies and learning strategies are presented as one of the main venues for helping to close the achievement gap. EPSB Theme Diversity (with specific attention to exceptional children including the gifted and talented, cultural and ethnic diversity) Assessment (developing skills to assess student learning) Literacy Education Closing the Achievement Gap Courses EDC 362, EDC 6341, EDC 777, EDC 730, EDC 746, EDP 658, EDS 558, EPE 773, EDL 770 EDC 634, EDC 777, EDC 730, EDC 746, EDS 558, EPE 773, EDL 770 EDC 634, EDC 777, EDC 730, EDC 746, EDS 558 EDC 634, EDC 777, EDC 730, EDC 746, EDP 658, EPE 773, EDL 770 57 Section 4: Faculty Information (Alphabetical Order by Program – Math, Science, MIC) Primary Secondary Science Education Faculty Person, J. Truman Stevens Primary Secondary Math Education Faculty Person, Margaret J. Mohr Faculty members responsible for professional coursework, clinical supervision, or administration in this program are included in the following table. Name Ronald Atwood Highest Degree, Field, & University Ph.D., Science Education, Florida State University Assignment and role Member of the MIC Program Faculty for Math and Science Faculty Rank Professor Scholarship, Leadership in Professional Associations, and Service Trundle, K. C., Atwood, R. K. & Christopher, J. E. A longitudinal study of conceptual change: Preservice elementary teachers’ conceptions of moon phases. Journal of Research in Science Teaching, Inpress Teaching or other professional experience in P-12 schools High School and Elementary School Teaching Experience Status (FT/PT to institution, unit, and program) FT, Curriculum and Instruction Trundle, K. C., Atwood, R. K. & Christopher, J. E. Preservice elementary teachers’ knowledge of observable moon phases and patterns of change in phases. Journal of Science Teacher Education, In press. Trundle, K. C., Atwood, R. K. & Christopher, J. E. Fourth grade elementary students’ conceptions of standards-based lunar concepts. International Journal of Science Education, In press. Frank Ettensohn Ph.D., Geology, University of Illinois at UrbanaChampaign Member of the MIC Program Faculty for Math and Science Professor Ettensohn, F.R., in press, The sedimentary manifestation of foreland-basin, tectophase cycles: Examples from the Appalachian Basin, U.S.A., in Mabesoone, J.M., and Neumann, V.H., eds., Cyclic development of sedimentary basins. FT, Department of the Earth and Environmental Science 58 Elsevier. Ettensohn, F.R., 2004, Modeling the nature and development of major Paleozoic clastic wedges in the Appalachian Basin, U.S.A.: Journal of Geodynamics, v. 37, 657-681. Ettensohn, F.R., Kasl, J.M., and Stewart, A.K., 2004, Structural inversion and origin of a Middle/Late Ordovician carbonate buildup: Evidence from the Tanglewood and Devils Hollow members, Lexington Limestone, central Kentucky (USA): Palaeogeography, Palaeoclimatology, Palaeoecology, v. 210, p. 249-266. David Helm Masters of Arts, Science Education and Rank I, Educational Leadership, University of Kentucky Instructor Secondary Science Methods Course, Member of Math and Science Program Faculty Instructor Science Teacher and Science Department Chair at Tates Creek H. S., Science Content Specialist, Fayette County Schools Jim Krupa Ph.D., Biology, University of Oklahoma Member of the MIC Program Faculty for Math and Science Associate Professor 2005 B. M. Graves, J. J. Krupa. Great Plains toad (Bufo cognatus) in Amphibian Declines: a Conservation Status of United States Species, M. Lannoo (ed.). University of California Press. ~ 15 Years, Biology and Physical Science Teacher; HS Certification in Biology and Chemistry PT FT, Department of Biology 2005 James J. Krupa, Terri A. Estes, Todd J. Crawford, Ann M. Schlosser, Kevin A. Chermak, Tiffany D. Justice, Devin L. Riggs, Bridget M. Larder, Justin A. Head, Heidi T. Schapker, and Joseph T. Forester. Impact of Fire on Small Mammals in a Mixedmesophytic Forest in Southeastern 59 Kentucky. Journal Kentucky Academy of Science. 66:67-70. 2001 J. J. Krupa, J. Workman, C. M. Lloyd, L. R. Bertram, A. D. Horrall, D. K. Dick, K. S. Brewer, A. M. Valentine, C. Shaw, C. M. Clemons, J. E. Clemons Jr., C. A. Prater, N. J. Campbell, S. B. Armold, N. J. Jones, and A. M. Clark. Distribution of the Allegheny Woodrat (Neotoma magister) in an isolated, mixmesophytic forest in Southeastern Kentucky. Journal Kentucky Academy of Science. 65(1):33-34. Denis Lester Master of Arts, Science Education and Rank I, Guidance and Counseling Clinical Supervision, and Instructor of Secondary Field Experience Class, Member of Math and Science Program Faculty ST Supervisor Writing Team, General Supervision Enhancement Grant (GSEG): activities for the standards in reading, mathematics and science that will be assessed, 2005 and 2006. Elizabeth A. E. Roland Masters of Arts, Science Education and Science Education Doctoral Candidate, University of Kentucky Ed.D., Science Education, University of Virginia Clinical Supervision, Member of Math and Science Program Faculty Graduate Assistant, ST Supervisor Chair: Department of Curriculum and Science; Chair of Math and Science Program Faculty; Methods Instructor; Member of MIC Program Faculty Graduate Assistant, Member of Math and Science Program Faculty J. Truman Stevens Jennifer Eli Master of Arts in Mathematics, University of Kentucky PT Presentations at Mid-western American Research Association, MidAtlantic Assoc. for Science Teacher Education, KY Science Teachers Association; KTIP Supervision ~30 Years, HS and MS Biology and Earth Science Teacher; HS Biology Certification 3 Years, HS Chemistry and Earth Science; HS Certification in Physical Sciences Associate Professor Teaching Line Graphs to Tenth Grade Students Having Different Cognitive Developmental Levels, Research in Science & Technological Education, 2003. (With S. Ates) 3.5 Years, HS Physics, Chemistry Teacher; HS Cert. in Physics and Chemistry FT, Curriculum and Instruction, Secondary Science Graduate Assistant Mohr, M. J., Goldsby, D., Kulm, G., Willson, V., Smith, D., Schroeder, D. C., & Eli, J. A. (2007, April). An assessment of mathematics knowledge for teaching: comparing elementary FT, Curriculum and Instruction, Middle and Secondary Science FT, Graduate Assistant 60 and middle grades preservice teachers. Paper submitted to the annual meeting of the American Educational Research Association, Chicago, IL. Schroeder, D. C., Mohr, M. J., Goldsby, D., Kulm, G., Willson, V., Smith, D., & Eli, J. A. (2007, March). An assessment of mathematics knowledge for teaching: comparing elementary and middle grades preservice teachers. Paper submitted to the annual meeting of the National Council of Teachers of Mathematics, Atlanta, GA. Eli, J. A. & Schroeder, D. C. (2006, June). Using technology to enhance mathematics understanding. Presented at the annual meeting of the Future Educators of America Workshop, Lexington, KY. Willis Johnson Ed.D., Mathematics Education, Temple University Member of the MIC Program Faculty for Math and Science Professor Program Chair for the National Council of Teachers of Mathematics Conference and Exhibition in Birmingham, AL, October 2005. 4 Years Math Teacher FT, Curriculum and Instruction Technology Coordinator, ACCLAIM (Appalachian Collaborative Center for Assessment and Instruction in Mathematics) sponsored by the National Science Foundation. 20022006. Attended the Annual Meeting of the National Council of Teachers of Mathematics (Philadelphia, 2004 and San Antonio, 2003) Bo Lankster Member of Math and Practicing 61 Science Program Faculty Carl Lee Ph.D., Mathematics, Cornell University Member of the MIC Program Faculty for Math and Science Professor Lee, C. W., 2004, Subdivisions and triangulations of polytopes, Handbook of Discrete and Computational Geometry, J. E. Goodman and J. O’Rourke, eds, CRC Press LLC, Boca Raton, second edition, pp. 383-406. Mathematics Teacher at Tates Creek HS HS Mathematics Teacher Certification FT, Mathematics Department Bayer, M. M., Lee, C. W., and Sturmfels, B., editors, 2004, The Louis J. Billera Birthday Issue, Discrete and Computational Geolmetry, 32. ACCLAIM (Appalachian Collaborative Center for Learning, Assessment and Instruction in Mathematics, National Science Foundation grant ESI-0119679 (5 years beginning September 2001; approximately $10M), co-PI. See www.appalmsp.org. Xin Ma Ph.D, Curriculum and Instruction, University of British Columbia Member of the MIC Program Faculty for Math and Science Professor Ma, X. (in press). Cognitive and affective changes as determinants for taking advanced mathematics courses in high school. American Journal of Education. FT, Curriculum and Instruction Ma, X., & Wilkins, J. L. M. (in press). Mathematics coursework regulates growth in mathematics achievement. Journal for Research in Mathematics Education. Ma, X., & Papanastasiou, C. (2006). How to begin a new topic in mathematics: Does it matter to students’ performance in mathematics? Evaluation Review, 30, 62 Richard Millman Ph.D., Mathematics, Cornell University Member of the MIC Program Faculty for Math and Science Professor Margaret J. Mohr Ph.D., Mathematics Education, Texas A & M University MIC Cohort Leader, Member of Math and Science Program Faculty, Member of MIC Program Faculty Assistant Professor 451-480. Dr. Millman is the Outreach Professor of Mathematics at the University of Kentucky, a tenured faculty in the Department of Mathematics created to support ongoing efforts of the Department of Mathematics to support pre-service and in-service teacher training for PreK-12 mathematics teachers, coordinate outreach activities to public schools throughout the Commonwealth, and advocate the cause of Mathematics Education in the Commonwealth of Kentucky. Mohr, M. J., Goldsby, D., Kulm, G., Willson, V., Smith, D., Schroeder, D. C., & Eli, J. A. (2007, April). An assessment of mathematics knowledge for teaching: comparing elementary and middle grades preservice teachers. Paper submitted to the annual meeting of the American Educational Research Association, Chicago, IL. Fulltime, Mathematics Department 1 Year, HS Math Teacher; HS Math Certification FT, Curriculum and Instruction Mohr, M. J., Binks, E., Shaw, B., Smith, D. L., & Smith, L. (2007, April). Using research-based literacy strategies to improve mathematics achievement in the middle grades. Paper submitted to the annual meeting of the American Educational Research Association, Chicago, IL. Mohr, M. J., Kulm, G., Goldsby, D., Smith, D., Willson, V., & Allen, G. D. (2007, April). An assessment of middle grades preservice teachers mathematics knowledge for teaching. Paper submitted to the annual meeting of the American Educational Research 63 Association, Chicago, IL. Craig Schroeder Masters of Arts, Mathematics Education and Science Education Doctoral Candidate, University of Kentucky Member of the MIC Program Faculty for Math and Science Graduate Assistant Mohr, M. J., Goldsby, D., Kulm, G., Willson, V., Smith, D., Schroeder, D. C., & Eli, J. A. (2007, April). An assessment of mathematics knowledge for teaching: comparing elementary and middle grades preservice teachers. Paper submitted to the annual meeting of the American Educational Research Association, Chicago, IL. 1 year Math Teacher FT, Curriculum and Instruction, Secondary Math Schroeder, D. C., Mohr, M. J., Goldsby, D., Kulm, G., Willson, V., Smith, D., & Eli, J. A. (2007, March). An assessment of mathematics knowledge for teaching: comparing elementary and middle grades preservice teachers. Paper submitted to the annual meeting of the National Council of Teachers of Mathematics, Atlanta, GA. Eli, J. A. & Schroeder, D. C. (2006, June). Using technology to enhance mathematics understanding. Presented at the annual meeting of the Future Educators of America Workshop, Lexington, KY. Elinor Brown Ph.D., Curriculum and Instruction, University of Akron Multicultural Strand for MIC Program, Member of MIC Program Faculty Associate Professor Brown, E. L. & Howard, B. (2005). Becoming culturally responsive teachers through service-learning: A case study of five novice classroom teachers. Multicultural Education, 12(4), 2-8. FT, Curriculum and Instruction Brown, E. L. (2005). Service-learning in a one-year alternative route to teacher certification: A powerful multicultural teaching tool. Equity 64 and Excellence in Education, 38(1), 61-74. Brown, E. L. (2004). What precipitates change in cultural diversity awareness during a multicultural course: The message or the method. Journal of Teacher Education, 55(4), 325-340. Les Burns Ph.D., English Education and Literacy, Michigan State University MIC Cohort Leader, Member of MIC Program Faculty Assistant Professor Burns, L. (2006). On being unreasonable: NCTE and political action in the age of accountability. Manuscript accepted for publication in English Education. 5 years experience in English language arts teaching for grades 9-12. FT, Curriculum and Instruction, Secondary English Education ~ 15 Years, HS English, Humanities, and Social Studies Teacher, Dean of English Department; HS Certification in English and Social Studies FT, Curriculum and Instruction, Secondary Education Burns, L. (2006). Effective teaching of appropriate language: A discourse analysis of English teacher preparation guidelines. Manuscript under review by Research in the Teaching of English. Burns, L., & Morrell, E. (2005). Critical discourse analysis in literacy research. 54th National Reading Conference Yearbook, 54, 2005. Mary Markowitz Ph.D., Secondary Education, University of Kansas MIC Coordinator and MIC Cohort Leader, Member of MIC Program Faculty Assistant Professor Markowitz, M. C. and Gut, D. M. (in progress). Examining self-Disclosure: What topics do college of education faculty disclose and why? Submitted to Teaching and Teacher Education, Fall2006. Markowitz, M. C. (in progress). A critique of charter school theory from a Deweyan perspective on democracy and education. Submitted to Educational Studies, Fall 2006 “A Critique of Charter School Theory 65 from a Deweyan Perspective on Democracy and Education,” The John Dewey Society, American Educational Research Association Conference, San Francisco, California, April 10, 2006. Joan Mazur Ph.D., Curriculum and Instruction, Cornell University Technology Strand for MIC Program; KTIP Supervisor; and Department Director of Graduate Studies, Member of MIC Program Faculty Associate Professor Mazur, J., Cole, H., Reed, D., and Claunch, D. (2005). An analysis of instructional practices and materials at the Just-4-Kids Safety Day Camps. Journal of Agricultural Safety and Health. 6 Years English Teacher; HS English Certification FT, Curriculum and Instruction Mazur, J. (2004). Conversation Analysis for Educational Technologists: Theoretical and Methodological Issues for Researching the Structures, Processes and Meaning of On-line Talk. In D. Jonassen (Ed.) Handbook of Research for Educational Communications and Technology. New York: McMillan. Mazur, J., and Lio, C. (2004). Learner articulation in an immersive visualization environment. In Proceedings of the Annual SIGCHI Conference, April 2004, Vienna, Austria. Rosetta Sandidge Ed.D., Vocational Education, University of Kentucky Discipline and Classroom Management Strand for MIC Program; Associate Dean of COE, Member of MIC Program Faculty Associate Professor Sandidge, R. F. (2005). Performancebased teacher education in Kentucky: Benefits and challenges at a research university. Proceedings of the Third Annual Hawaii International Conference on Education, Honolulu, HI, January. FT, Curriculum and Instruction; Associate Dean COE Sandidge, R. F. (2004). Preparing quality teachers: Standards-based teacher education in Kentucky. In Proceedings of the Conference: 66 Developmentally Appropriate Teaching Practices (pp. 89-113). Taipei, Taiwan: Taipei Municipal Teachers College and the Taiwan Association for Educator Professional Development. Sandidge, R. F. (2003). A response to “Teacher recruitment: Trends and challenges for Kentucky’s school reform agenda” by Paul A. Winter. In R.N. Ronau & R. Shapiro (Eds.), Setting a Research Agenda for Educational Reform and Improvement (pp. 99-101). Lexington/Louisville, KY: University of Kentucky/University of Louisville Collaborative Research Conference. 67 University of Kentucky Department of Curriculum and Instruction Curriculum Contract Master’s Degree in Education with Initial (Rank II) Certification Please TYPE or PRINT Name (first) (last) Social Security # Address (mi / maiden) Preferred Name E-Mail Street City State Zip Phone Home Work Other Date of Admission to MIC Program Subject Area Cohort (certificate grade level specified for each cohort) Business & Marketing Education (grades 5-12) English Education (grades 8-12) Science Education – Biology (grades 8-12) Social Studies Education (grades 8-12) Science Education – Chemistry (grades 8-12) Mathematics Education (grades 8-12) Science Education – Earth Science Science Education – Physics Student Fully Admitted to Program (see admissions overview materials) Comments, including Specification of Standards Deficiencies. Required Coursework (completed in fall and spring) Course Title Completed Elective Coursework (6 hours in subject area, 3 in Curriculum & Instruction Course Title Completed (24 credit hours) Grade Credits (9 credit hours) Grade Credits 3 3 3 33 Total Credit Hours 68 Successfully complete mid-point assessment review, including presentation of required assessment materials. Comments: Successfully complete exit assessment review, including presentation of exit portfolio, successful student teaching evaluation, and successful completion of any required on-demand tasks. Comments: 3.0 minimum GPA earned in all MIC coursework Successfully complete the master’s examination Applied for Master’s Degree with Graduate School Program Area To be eligible for a recommendation for certification, candidates must successfully complete the appropriate PRAXIS II Specialty Area Examination(s) and the Principles of Learning and Teaching Examination. The required examinations and required cut scores are identified, by discipline, in the next section. Required PRAXIS II Examination Required Passing Score 161 5-12 Principles of Learning and Teaching, Grades 5-9 (0523) or Grades 7-12 (0524) Business Education (0100) English Education, 8-12 Principles of Learning and Teaching, Grades 7-12 (0524) 161 English Language, Literature and Composition: Content Knowledge (0041) 160 English Language, Literature, & Composition: Essays (0042) Principles of Learning and Teaching, Grades 7-12 (0524) 155 Mathematics: Content Knowledge (0061) 125 141 Science Education: Biology, 8-12 Mathematics: Proofs, Models, & Problems, Part 1 (0063) Principles of Learning and Teaching, Grades 7-12 (0524) 146 Science Education: Chemistry, 8-12 Biology: Content Knowledge (0235) Principles of Learning and Teaching, Grades 7-12 (0524) 147 Science Education: Earth Science, Chemistry: Content Knowledge (0245) Principles of Learning and Teaching, Grades 7-12 (0524) Earth Science: Content Knowledge (0571) Principles of Learning and Teaching, Grades 7-12 (0524) 145 Physics: Content Knowledge (0265) 133 Principles of Learning and Teaching, Grades 7-12 (0524) 161 Social Studies: Content Knowledge (0081) 151 Social Studies: Interpretation of Materials (0083) 159 Business and Marketing Education, Mathematics Education, 8-12 8-12 Science Education: Physics, 8-12 Social Studies Education, 8-12 590 161 161 161 161 161 Note: 1. 2. Student must attach signed copies of all subject matter course listing forms demonstrating completion of all courses with a minimum GPA of 2.50 on each form. Student must demonstrate continued adherence to the Kentucky Professional Code of Ethics and have a signed Declaration of Eligibility for a Certificate on file. Student Signature Date Advisor Signature Date Kentucky educator certification requirements are subject to change. Before registering for the test(s), please check the Education Professional Standards Board website at www.kyepsb.net for current test requirements and current cut scores. You may also contact Ms. Jaime Rice at 502-564-4606 or toll free at 888-598-7667. To receive a UK recommendation that you are eligible for a state educator certificate, you must have taken the Kentucky EPSB required examinations and met the Kentucky EPSB cut score requirements. 69