water sampling
advertisement

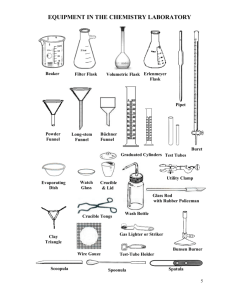
Water Sampling Techniques Chemistry 130, Fall 2006 Water Sampling Procedures Perform a visual inspection of the site. Please note the following aspects: 1) 2) 3) 4) Potential pollution sources (industrial, agricultural, domestic, animals) Condition of water (clear, cloudy, color, smell, foam) Map of Site (electronic or sketched, photograph if possible) Brief written description of site. Samples and Controls: 1) Sample all sites in triplicate. 2) Collect all samples in 100mL or 1L Whirlpak bags 3) Use a field blank. This consists of taking one of Whirlpak bags, filled with deionized water, along to the sampling site. The bag is opened for roughly the same amount of time it took you to sample. The bag is then reclosed and returned to evaluate with your samples. 4) Use a lab blank. 5) All samples must be clearly labeled by site, number of sample, and status as field blank (FB) or lab blank (LB). For example, say you sampled Miller Creek at the bridge and in the marsh. You would generate the following sets of samples: MCB1, MCB2, MCB3, MCBFB; MCM1, MCM2, MCM3, MCMFB; MCLB. Use an indelible marker to label all bags before trying to take sample (wet plastic does not take ink well). In addition to the sample IDs, all samples should be dated.. Sampling Procedure: 1) If sampling a body of running water, point the mouth of the bag upstream and your hands downstream to avoid contamination. 2) If sampling from a water faucet, run the faucet for 1 minute before obtaining a sample. 3) Rinse the bag twice with the sample water prior to filling and closing. 4) Fill bag as full as possible. Half-filling the bottle leaves more room for oxygen which will promote degradation of your sample. 5) Collect data such as temperature and pH which affect the solubility of many ions. Preserving and Storing your Sample: 1) Storage before filtration should be at 4 °C for not more than 2 days. 2) Storage after filtration should be at 4 °C for not more than 30 days. 1 Filtration of the sample through 0.45 μm filters removes most bacteria and will slow sample degradation. All glassware used for filtration should also be cleaned using the acid washing steps delineated below. Note that the controls should be filtered in exactly the same manner as the samples. (For this project, we will consider these guidelines to obtaining the best data. If you deviate from the preservation and storage guidelines, you will not be penalized. It is critical that you correctly report how your samples were actually handled so we can best understand your analyses.) Cleaning of glassware used in analysis Acid Washing – (Safety Goggles Required!!) This step serves to oxidize and solubilize any oxidizable materials. Rinse the inside of the glassware with 8 M nitric acid (HNO3). 5 to 20 mL acid should suffice. It is not necessary to fill the glassware, just add enough that you can turn the glassware and wet all internal surfaces. If the glassware is not visibly dirty and the acid does not discolor, you can reuse the acid for other glassware. Rinse the bottle twice with tap water. Do not reuse the rinse water. Rinse the inside of the glassware with 1.2 M hydrochloric acid (HCl) once again being sure to wet all internal surfaces. This acid wash can be reused if not visibly discolored. Rinse with tap water three times. Do not reuse the rinse water. Do a final rinse with three individual portions of deionized water. Do not reuse the rinse water. Do not dry the internal surfaces or touch them with your hands. All Acid Must Be Disposed of in the Acid Waste Jars Provided! Do Not Pour it Down the Drain! Pay special attention to avoiding contact with your hands around openings of the glassware. Reduce accidental contamination of the glassware exterior which could then be cross-contaminated to the contents during later handling. These guidelines are based on a more rigorous procedure outlined in Environmental Science and Technology 1987, 21, 749. Another excellent source consulted was The Chemistry of Water by Kegley and Andrews. 2