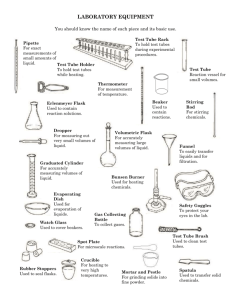
Sequencing Reaction

Sequencing Rxn 1 of 2
Sequencing Reaction
(following miniprep of plasmid DNA)
A forward and a reverse reaction is to be prepared in the following manner:
Forward Reaction:
1.
Sterilized SDW***
2.
Big Dye buffer*
3.
Forward Primer*
4.
DNA template (after miniprep)
5.
Big Dye Terminator Mix**
6.
TOTAL VOLUME tube__
__ul
4 ul
2 ul
__ul
1 ul
20ul tube__
__ul
4 ul
2 ul
__ul
1 ul
20ul tube__
__ul
4 ul
2 ul
__ul
1 ul
20ul
* located in -20˚C in box labeled “Universal Primers”
** located in -20˚C/top fridge labeled “PCR sequence” – brown tube labeled “BDT” tube__ etc.
__ul
4 ul
2 ul
__ul
1 ul
20ul
*** Add SDW up to 20 ul
Reverse Reaction:
7.
Sterilized SDW
8.
Big Dye buffer
9.
Reverse Primer
10.
DNA template
11.
Big Dye Terminator Mix
12.
TOTAL VOLUME
Sequencing Reaction Conditions:
1. 96C 2 min
2. 96C 30sec
3. 50C 15sec
4. 60C 4 min
5. Goto Step 2 Repeat 24X tube__ tube__ tube__ tube__ etc.
__ul
4 ul
2 ul
__ul
1 ul
20ul
__ul
4 ul
2 ul
__ul
1 ul
20ul
__ul
4 ul
2 ul
__ul
1 ul
20ul
__ul
4 ul
2 ul
__ul
1 ul
20ul
6. Hold at 15C
NOTE:
1.
Big Dye Terminator mix is obtained from Denise (~100ul), and 2ul was aliquoted into small PCR tubes…prepared on ice.
2.
Make a Master mix containing SDW, Big Dye buffer and Forward Primer for
Forward reaction and Reverse Primer for Reverse reaction. Make 1 master mix for
Forward reaction and the other for Reverse Reaction (account for one extra tube). E.g.
You want 10 forward reaction samples, prepare for 11 samples…that would make:
SDW = 11ul x 11
Big Dye buffer = 4ul x 11
T7 Promoter (forward primer) = _2ul x 11
Total 187ul/11 = 17ul in each tube
*** Remember 2ul of BDT mix already aliquoted in tubes and 1ul DNA added last!
Revision 01/20/07
Sequencing Rxn 2 of 2
Similarly, prepare master mix for reverse reaction as well……..exact same except for the reverse T7 Terminator primer……
3.
Big Dye Terminator Mix is already prepared in aliquots
4.
Add DNA last in individual tubes
Purify Sequencing Reaction
1.
Take purification tubes out.
2.
Clean purification tubes 2X by adding 700 ul SDW and centrifuge 1 min @ max speed.
3.
Discard flow through and flip tube over to get rid of previous column residue.
4.
Get Sephadex (in 4˚C) and swirl it to mix. Add 700 ul Sephadex and centrifuge for
2 min @ 2 rpm! Sephadex form the column.
5.
Get one 1.5 microcentrifuge tube per each purification tube use and label accordingly.
6.
Insert the inner tube of the purification tubes into appropriate 1.5 microcentrifuge tubes.
7.
Spin sequence rxn product.
8.
Directly pipette each sequence rxn (~20 ul) product into appropriate column. Do not pour on top of broken column.
9.
Centrifuge at 2 rpm for 2 min.
10.
Save eluted sequence DNA(s) in 1.5 microcentrifuge tubes to dry in room N210
(Autoclave Room). Follow instruction on the wall. Let dry for 25 mins.
11.
Meanwhile, put the inner purification tubes back into the outer tubes and wash 2X with 700 ul SDW.
12.
Collect DNA samples in tube and: a.
Give to Denise to sequence the DNA b.
If she is not there, leave in -20˚C (your box) and give it to her when she is present.
Revision 01/20/07