Sample lab report from 2006 - Department of Environmental Sciences
advertisement

December 8, 2006
Dr. Lisa Totten
Atrazine lab report
The Assessments of Atrazine in the Raritan River, rain, tap and well water samples
through Solid Phase Extraction and High Performance Liquid Chromatography
Abstract:
The amount of Atrazine found in the Raritan River (5.82 ug/L) and the rainwater
(1.12ug/L) samples collected in New Brunswick were expected. The Raritan River results
show that there is a large percent of Atrazine that is not absorbed into the ground, so
when it rains the Atrazine is carried from the farm land into the waterways. The
Rainwater samples showed that there is a significant amount of Atrazine that is
evaporated into the atmosphere when the farmers spray their crops. The amount of
Atrazine found in the wells and the tap were not significant because they were under the
Environmental Protection Agency limits (EPA). There were natural and man made filters
that was able to cleanse the well and tap water samples of Atrazine. Thus making the
amount of Atrazine found in the samples much lower than the Raritan and the rain water
samples. The methods used in the extraction of Atrazine were Solid Phase Extraction
(SPE) and High Performance Liquid Chromatography (HPLC). The Atrazine resides in
the water used in the samples through the HPLC method using the SPE. The purpose of
the experiment is to measure the amount of Atrazine that is run off and exposed tot eh
atmosphere through the process of spraying crops with a herbicide.
Introduction:
Atrazine is herbicide, which is put down before the plants grow, that is use to
battle broadleaf weeds and some grassy weeds in cornfields. The way atrazine works as a
herbicide is that it inhibits the photosynthesis process. There is an “estimated amount of
76.4 million pounds that are applied annually to farmlands. Usage on corn accounts for
approximately 86% of total U.S. domestic usage (in pounds), followed by sorghum at
10% and sugarcane at 3% (all other uses take up the remaining 1%). Approximately 75%
of the field corn acreage grown in the U.S. is treated with Atrazine” (2). In water,
Atrazine has the ability to metabolize into “2 main dealkylated chloro metabolites:
sethyldesisopropylatrazine (DEDIA) and desethylatrazine (DEA). In soils, the main
metabolites found are 2-hydroxyatrazine (HA), hydrohydroxyterbutylazine (HT),
desethylhydroxyatrazine (DEHA), and desisopropylhydroxyatrazine (DIHA)” (1). The
samples tested are tap, rain, well and Raritan River water samples. A well water sample
was collected from Dr. Lisa Totten’s home in Bridgewater, New Jersey. Rainwater
samples were collected in Bridgewater, New Jersey. There was a sample collected from
the Raritan River and a sample of tap water collected at the Environmental Science and
Natural Resource building. All the samples were collected in November 2002. Through
the measurement of Atrazine, using a Solid Phase Extraction (SPE) step and High
Performance Liquid Chromatography (HPLC), in the samples we are able to assess the
amount of Atrazine that is actually absorbed into the ground and how much is washed off
the soil when it rains. In a HPLC method, “it utilizes a liquid mobile phase to separate
the components of a mixture. These analytes are first dissolved in a solvent, and then
forced to flow through a chromatographic column under a high pressure. In the column,
the mixture is resolved into its components. The amount of resolution is important, and is
dependent upon the extent of interaction between the solute components and the
stationary phase” (1). Atrazine is a very polar compound therefore it is possible to find
them in animal tissues, soils and water. The Environmental Protection Agency (EPA)
does not consider Atrazine to possess any carcinogenic possibilities. If Atrazine were to
enter humans there is no acute and chronic dietary risk from food. But if Atrazine did
enter the ecosystem if is “acutely toxic to freshwater fish and highly acutely toxic to
aquatic invertebrates. Atrazine is chronically toxic to fish and aquatic invertebrates” (2).
The purpose of this experiment is to measure the amount of Atrazine in the rain, well, tap
and Raritan River water samples to assess the amount of Atrazine that washed off the soil
into the waterways.
Procedure:
Setting up the SPE:
Set up the SPE manifold with test tubes and the Lichrolut EN from Merck extraction
cartridges. Rinse the SPE cartridges with 5 ml of methanol and then 5 ml of ethyl acetate.
After discarding the waste methanol and ethyl acetate at the bottom of the manifold
reassemble the manifold with no rack inside. After rinsing the cartridges with 5 mL of
water prepare to run the sample through the cartridge by measuring the correct amount of
sample into a graduated cylinder. Run the samples through the SPE cartridges. After the
samples are completely extracted, place a small piece of filter paper over the opening of
each SPE cartridge, and continue to apply vacuum to the cartridges until they appear dry
or if not dry after 15 minutes discontinue the vacuum process. Open the manifold and
discard the water. After rinsing the test tubes with ethyl acetate label the samples and
reassemble the manifold with the rinsed test tubes. Rerinse the test tubes with 5 ml of
methanol and then 5 ml of ethyl acetate and transfer the eluent into the conical-bottom
flasks. The samples were not rotary evaporated till dryness. Instead they were dissolved
in a phosphate buffer (no acetonitrile) and weighed to determine the volume (assuming
density = mg/mL)
High Performance Liquid Chromatography:
The HPLC system was from Beckman-Coulter and consisted of a 125 Solvent Delivery
Module (pump), AS 508 auto sampler, and 168 Diode Array detector. Prepare a phosphate
buffer at (.005 M) at pH 7.2.Assemble the HPLC system with SpherisorbS5 ODS2 column
(or equivalent). Attach acetonitrile as solvent A and phosphate buffer as solvent B. Prime
each pump.
.
Program the HPLC as follows:
Diode array detector: analyze at 210, 220, 230 and 245 nm, recorded in the 190–400 nm
range. To set up the pump set up the linear gradient from 2 to 90% of solvent A in 60
min. Flow-rate = 1 ml/min. Run an atrazine standard curve before each set of samples.
Using the peak areas from 220 nm, quantify the amount of atrazine in each sample, using
the following calculation. A sample calculation of how the Mass of Atrazine is below.
The mass of Atrazine in the Raritan River is trying to be determined.
Massatz = {( Areaatz/AreaIS ) x Mass IS } / RRF
1.45 ug= ((21179/1461432)*6.10 )/241826.0
Divide the mass of atrazine in each sample by the original volume of sample run through
the SPE cartridge.
1.45ug/ 250 L= .0058 ug
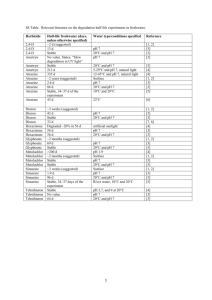
Results:
Graph 1:
standard curve
1600000
y = 241826.06584362100000x
R2 = 0.99975861442089
1400000
area counts
1200000
1000000
800000
600000
400000
200000
0
0
2
4
6
8
m g/L atrazine
Graph 1 represents the area of the Atrazine peaks vs. the amount of Atrazine
concentration found in each sample. Since the graph was forced through zero the graph’s
R^2 value is going to be very close to 1. The R^2 value is close to 1 this means that the
results are reproducible. For standard curve, determine the relative response factor (RRF)
by plotting the ratio of the area of Atrazine to the peak area of the internal standard
(areaatz/ areaIS) and the ratio of the mass of Atrazine to the mass of the internal standard
(massatz/massIS). Perform a linear regression. The slope is the relative response factor
(RRF). The RRF value is 241826.0. The RRF value was obtained from the slope of the
line.
Chart 1:
Matrix
samples =>
Area counts =>
Conc (mg/L)
rain
well
Raritan
Tap
Spike
53905
0
211719
0
1461432
0.27
0.05
0.92
0.05
6.04
volume of extract (injected on HPLC)
(mL)
1.02
1.01
1.58
1.04
1.01
mass (ug) in extract (injected on HPLC)
0.28
0.05
1.45
0.06
6.10
originally extracted volume (mL)
250
250
250
250
100
concentration in the water sample (ug/L)
1.12
0.22
5.82
0.22
From Chart 1 the amount of Atrazine in each sample can be found in each sample. The
amounts of Atrazine found in the well and tap water, 0.22ug/L, was not significant
enough to cause a peak from the HPLC. There were significant peaks of Atrazine in the
Rain and Raritan water samples. In the Rain water there was 1.12ug/L and in the Raritan
River sample there was 5.82ug/L found. These results mean that there was a significant
amount of runoff and evaporation, into the atmosphere, from the farms lands that used
Atrazine. For quality control methods a Matrix Spike was used to help keep track of the
samples going through the HPLC system. A Matrix Spike was used to test for the anions
that are being tested for in the samples. There is a large concentration of Atrazine tested
to see what the peak would look like before the actual samples are tested. Blanks were
also run through the system to make sure the HPLC was clean before running the samples
to avoid contamination of the samples.
Conclusions:
The results determined were expected from the samples. The Raritan River and
the rainwater sample had significant traces of Atrazine in them (>1ug/L). A major reason
for the concentrations of Atrazine being so significant in the waterways is that when
farmers spray the Atrazine onto their crops to prevent weeds from growing, a significant
amount is released into the atmosphere and run off the land into the waterways. The
concentrations in the river and the rain are able to show that not a lot of the Atrazine is
actually absorbed into the ground. The River and Rainwater samples did not meet
standards as drinking water status, .003mg/L (2). The samples take from the tap system at
Rutgers and the well water samples did not have a large amount of Atrazine (<1 ug/L)
because there are filtering process that are able to filter out the Atrazine. In the ground
well water is naturally filtered by the sands and other materials in the ground and above
ground all drinking water is filtered before it can be distributed to the community. The
well and tap water samples were under the Environmental Protection Agency limits. The
water from the tap and the well are consumable by humans (2). The results from the
natural waterways and the drinking water supply were normal. Atrazine is not a very
polar substance. It prefers to be in a dissolved phase, which allows for the movement of
the Atrazine through waterways and through the atmosphere. The experiment went as
expected. The only abnormal result was the recovery being over 100%. This was possible
through human error of measuring the amount of Atrazine before running the samples or
the machine being contaminated before usage. To further any studies of atrazine it is
possible to study the flow of atrazine during a storm. The storm water run off is important
because that is when the highest concentration of Atrazine is picked up from the land and
transported. The experiment was able to show what was expected of atrazine levels in
the waterways and drinking waters. The waterways were expected to have higher levels
of atrazine since they are not treated while the drinking waters were under the EPA’s
limit.
References:
(1) Carabias-Mart´ıneza, Rita, et al. Determination of herbicides and metabolites by
solid- phase extraction and liquid chromatography Evaluation of pollution due to
herbicides in surface and groundwaters. Journal of Chromatography A. December
2001, 950 (2002) 157–166.
(2) Environmental Protection Agency, Atrazine. CAS # 1912-24-9, Agency for Toxic
Substances and Disease Registry (ATSDR): September 2003.
Acknowledgements:
Thank you to Yi Tan for setting up and taking down the lab experiments and Dr. Lisa
Totten for allowing us the chance to perform the experiments in the lab. I also want to say
thanks to my lab partners for all their help and support with all.