Homework #4
advertisement

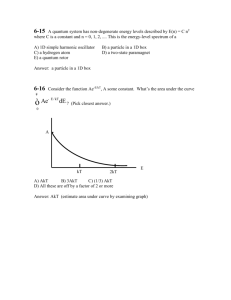
CHEM 614 1. HOMEWORK 4 2002 For a macroscopic object of mass 1.0 g moving with speed 1.0 cm s-1 in a one- dimensional box of length 1.0 cm, find the quantum number n. 2. When a particle of mass 9.1 x 10-28g in a certain one-dimensional box goes from the n = 5 level to the n = 2 level, it emits a photon of frequency 6.0 x 1014 s-1. Find the length of the box 3. (a) Sketch rough graphs of and of 2 for the n = 4 and n = 5 particle-in-a-box states. (b) Use calculus to find the slope of the n 4 2 curve at x l / 2 and check that your curve was drawn with the correct slope. 4. True or False? (a) The particle-in-a-box ground state has quantum number n = 0. (b) The particle-in-a-box stationary-state wave functions are discontinuous at certain points. (c) The first derivative of each particle-in-a-box stationary-state wave function is discontinuous at certain points. (d) The maximum probability density for every particle-in-a-box stationary-state is at the center of the box. (e) For the particle-in-a-box n = 2 stationary state, the probability of finding the particle in the left quarter of the box equals the probability of finding it in the right quarter of the box. (Answer this and the next question without evaluating any integrals.) (f) For the n = 1 particle-in-a-box stationary state, the probability of finding the particle in the left third of the box equals the probability of finding it in the middle third. (g) The wavelength of the particle-in-abox absorption transition from quantum number n to n + 1 decreases as the value of the quantum number n increases. 5. If  f ( x) 3x 2 f ( x) 2 x df / dx , give an expression for  . 6. ˆ x ex . Give three different operators  that satisfy Ae 7. Verify the commutator identity [ Aˆ , Bˆ ] [ Bˆ , Aˆ ] . 8. If  is linear, show that ˆ cAg ˆ Aˆ (bf cg ) bAf where b and c are arbitrary constants and f and g are arbitrary functions 8. Which of the following functions are eigenfunctions of d2/dx2? (a) ex; (b) x2; (c) sin x; (d) 3 cos x; (e) sin x + cos x. Give the eigenvalue for each eigenfunction. 9. If is a normalized wave function, what are its SI units (kg/m/s) for (a) the one-particle, one-dimensional case; (b) the one-particle, three-dimensional case; (c) the n-particle, threedimensional case? 10. A classical harmonic oscillator with the mass of 1.00 kg and the operating with a force constant of 2.00 kg s-2 = 2.00 J m-2 is released from the rest at t = 0 and x = 0.100 m. (a) What is the function x (t) describing the trajectory of the oscillator? (b) Where is the oscillating mass when t = 3 seconds? (c) What is the total energy of the oscillator? (d) What is the potential energy when t = 3 seconds? (e) What is the time-averaged potential energy? (f) What is the time-averaged kinetic energy? (g) How fast is the oscillator moving when t = 3 seconds? (h) Where are the turning points for the oscillator? (i) What is the frequency of the oscillator? 1 2 kx for 0, and for x<0. (a) Sketch V(x), 2 (b) what is the boundary condition at the infinite boundary? (c) What can you say about the eigenfunctions and eigenvalues for this system? 11. In the harmonic oscillator, suppose V (x)= 12. In the harmonic oscillator, if V = E/2, what is the average potential energy for a harmonic oscillator when n = 5? What is the average kinetic energy? 13. Calculate the force constants for vibration in H19F, H35Cl, H81Br, and H127I, given that the infrared absorptions for the n = 0 to n = 1 transitions are seen, respectively, at 4138, 2991, 2649, and 2308 cm-1. What do these force constants imply about the relative bond strengths in these molecules? 14. Referring to Module 4, (a) show for yourself how equation 4.10 is arrived at from 4.8. (b) Convince yourself by sketching the first six wavefunctions that equation 4.16 is correct. 15. Write a brief paragraph about the following topics (a) Postulate 5 (b) The superposition Principle (c) Expectation Value of an Operator (d) Hermitian operators (e) The specification of states (f) Commutators (g) Complementarity and Uncertainty.