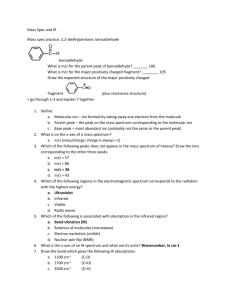
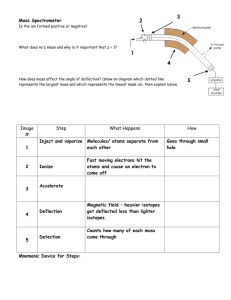
Mass Spectra - Chemical Paradigms
advertisement

Mass Spectra Lab Aim: Interpret the mass spectra of mercury, chlorine and krypton and calculate their relative atomic masses using two different measuring devices. IB Assessment: Data Collection and processing Conclusion and evaluation See below for detailed requirements A. Mass spectrum of the element Mercury, Hg Present the following data for each fragment: 1. Isotope fragment as indicated on the spectrum and written in notation AX+1 2. Charge of fragment (all +1 charges) 3. Mass / charge ratio = mass of positive ion ÷ charge of positive ion 4. Total sum of mass / charge 5. Peak height in mm 6. Total sum of the peak heights in mm 7. Fragment = mass/charge x peak height 8. Total sum of fragments 9. Ar (Hg) = Total sum of fragments ÷ total sum of peak heights 10. % error using the Ar(Hg) from the periodic table as the theoretical / literature / accepted value. % Error = │(Experimental - Accepted) ÷ Accepted│ x 100 B. Mass spectrum of the element Krypton, Kr Present the following processed data for each fragment: 1. Isotope fragment as indicated on the spectrum and written in notation AX+1 2. Charge of fragment (+1 charges) 3. Mass / charge ratio = mass of positive ion ÷ charge of positive ion 4. Total sum of mass / charge 5. Peak height in mm 6. Total sum of the peak heights for +1 ions in mm 7. Fragment = mass/charge ions x peak height 8. Total sum of fragments for +1 ions 9. Ar (Kr) = Total sum of fragments for +1 ions ÷ total sum of peak heights for +1 ions. 10. % error using the Ar(Kr) from the periodic table as the theoretical / literature value. NOTE: Only the +1 peaks are incorporated to the data analysis to improve the accuracy because the +2 peaks are smaller and harder to read decreasing the statistical validity of the measurements. 1 C. Mass spectrum of the element Chlorine, Cl2 Present the following processed data for each fragment: 1. Isotope fragment as indicated on the spectrum and written in notation AX+1 2. Charge of fragment (+1 charges) 3. Mass / charge ratio = mass of positive ion ÷ charge of positive ion 4. Total sum of mass / charge 5. Peak height in mm 6. Total sum of the peak heights in mm 7. Fragment = mass/charge ions x peak height 8. Total sum of fragments 9. Ar (Cl) = Total sum of fragments for +1 ions ÷ total sum of peak heights for +1 ions. 10. % error using the Ar(Cl) from the periodic table as the theoretical / literature value. NOTE: Only the +1 peaks are incorporated to the data analysis to improve the accuracy because the +2 peaks are smaller and harder to read decreasing the statistical validity of the measurements. The Cl2 fragments are not used for the Ar determination because this is based on the mass of an atom, not a molecule. Treatment of significant figures In the IB you are not generally penalized for having one too many significant figures in answers to calculations, unless the question specifically states the correct number of significant figures is required. Answers with fewer significant figures will. In all calculation done in this class the assumption is that significant figure rules will be followed, regardless of whether this is stated or not. As a general rule measured numbers are used in the significant figure count but counted numbers, integers (atomic masses, moles in a formula) and units conversions are not. When carrying out multiple step problems keep one extra significant figure throughout the whole problem, to reduce the random error due to rounding. The final result should be consistent with the number of significant figures given in the experimental measurements. Points are awarded for process so all problems need to show the following steps: 1. Formula with subject 2. Number substituted into the formula 3. Answer with correct units and significant figures. Units for quantities also need to be shown. The omission of units or the use of incorrect units will be penalized. Where appropriate write answers in scientific notation. D. Data Comparison Prepare a table to summarize the relative atomic masses and percentage errors obtained using a ruler and digital caliper. 2 Assessment Rubric for Mass Spectrum Lab Scoring rubric: completely met = 2 ; partially met = 1 ; not met = 0 Recording Raw Data All units of measurement and absolute uncertainties are recorded. Column headings in tables are clearly labeled and include units and uncertainties in measurement. Raw quantitative data is presented in easily interpretable, organized and labeled table(s). A well organized table will: borders/lines around text and numerical data not run over two pages columns to be compared placed next to one another be called a table and numbered consecutively have a concise descriptive title that relates the measured (dependent) and changed (independent) variables on top of the table text and data centered rows and columns are evenly distributed 11–12 point font size and a consistent font type for electronic tables correctly placed in document. Consistent and correct use of decimal places and/or significant figures so that there is no variation in the precision of the raw data. The level of precision should be consistent with that of the raw data. Processing Raw Data Complete and correct quantitative analysis of the data is carried out. This could include combining, manipulating raw data to determine the value of an answer, or taking the average of several measurements and transforming the data into a form suitable for graphing. At least one sample per calculation showing the steps involved. Identical calculations do not need to be repeated. Presenting Processed Data Processed data for both the ruler and the caliper are summarized in a table or in some other easily interpretable manner so that all the stages to the final result can be followed. Raw and processed data can be incorporated into the same table. Significant figures and units are used correctly in calculations and for presenting the numerical results of processed data. One extra significant figure can be kept throughout the entire calculation to reduce rounding errors. The final result is consistent with the number of significant figures in the experimental measurements and any calculations based on them. 3 Conclusion and Evaluation The actual results are compared to the expected results (literature) by calculating the % Error. The source of the literature value should be stated. % Error = │(Experimental – Accepted) ÷ Accepted│ x 100 If appropriate the expected (from the hypothesis) and actual results are compared. It is stated whether the results supported or disproved the hypothesis Comment on the difference between the % Error for the relative atomic masses obtained with the ruler and digital caliper. Describe the sources of systematic error and/or random encountered and difficulties with the control of variables and how these affected the precision, accuracy and reproducibility of the results. Describe the effect of any of the identified errors on the magnitude and direction (result too high or too low) on the final result. In other words did the error cause the result to be lower or higher than expected? Suggest feasible ways the method could be improved so the results will be closer to what is expected. These modifications should address ways to reduce the systematic errors and/or random described above, reduce approximations or provide better procedures for measurement. Improvements should be specific (not vague), realistic and lead to significant improvement in experimentation. They should not involve unavailable equipment or materials. General Directions Ideas are expressed concisely. The reports is organized allowing for easy interpretation and written using the passive voice. Instead of: We measured 50 ml of HCl (aq) acid in a 100 ml cylinder, use: 50 ml of HCl (aq) was measured in a 100 ml cylinder. Pronouns [I, we, us, you] are replaced with “the”. Chemical substances are named correctly and vague nouns like “it”, “the substance”, “they” are avoided. Author’s name, date practical work was completed in class and name of partner/s, IB SL/HL on top right hand corner. Pages are numbered with 1.5 spacing between bodies of word processed text. 4