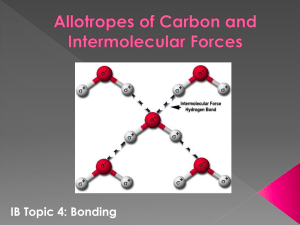
When an ion and a polar molecule (a molecule that has a dipole
advertisement

When an ion and a polar molecule (a molecule that has a dipole moment) get near one another, the charged species (the ion) will interact with the partial charged species (the polar molecule) such that the oppositely charged and partial charged ends will attract one another. The most common and probably the most important example is the dissolving of an ionic compound (ions) in water (a polar molecule). The ionic compound will break down into ions which will be attracted to either the partially negative oxygen or the partially positive hydrogens in the water molecule. When polar molecules lie near one another, they will orient themselves in such a manner that their oppositely charged ends are near one another. In a solid, the molecules will lock into place with their oppositely charged ends attracting one another. In a liquid, again the oppositely charged ends will attract one another but the molecules are allowed to move and flip. They are not locked into place as in a solid, instead, it is like a square dance. They are “partnered up” but constantly flip and roll around, finding new molecules to interact with. 1 Dipole-dipole forces are very important for explaining some common physical properties – such as boiling point of molecules. For molecules of similar molecular weight, the greater the dipole moment, the larger the disparity in the partial charges (the more “ionic in nature the bond becomes), the greater the forces between the molecules and the more energy it takes to overcome those attractions. Thus, the greater the dipole moment, the more energy is needed to boil the compound (the higher the boiling point). This means, that when given the dipole moments, you can predict the order of when the compounds will boil! Concept Test Put the following species in order of increasing boiling point based on their dipole moments, put the lowest boiling point on the LEFT and the highest boiling point on the RIGHT Molecule CH3CH2CH3 CH3Cl CH3OCH3 CH3CN CH3CHO Molecular Wt (g/mole) 44.09 50.48 46.07 41.05 44.05 Dipole Moment (D) 0.1 1.9 1.3 3.9 2.7 The special ability of a molecule to form a hydrogen bond only occurs when a hydrogen is bonded to a highly electronegative atom that has lone pairs – meaning an oxygen, nitrogen, or fluorine. ONLY!!! The N-H, O-H, and F-H bonds are very polar, so the electron density is drawn away from the hydrogen (if we calculated oxidation number on the H – what would it be for every situation like this??) As a result, this partially positive hydrogen of one molecule is attracted to the partially negative lone pair on a N, O, or F on another molecule. The hydrogen bond is shown with a dashed or dotted line between the H and the other atom (O, N, or F). It is not a complete bond – it is the interaction and attraction of oppositely partially charged species! 2 As long as the N, O, or F have lone pair electrons and there is a partially positive H, H bonds can form. Concept Test Which of the following molecules will form H-bonds? H2N-C≡N CH2=N=N N = N CH2 H bonding is another force that must be overcome in order to change phases. It keeps the molecules together, specifically in the liquid state where molecules are free to move around and interact. When comparing boiling points, if a molecule can H-bond, this extra force that must be overcome will cause an enormous deviation because the H bonds require additional energy to break before the molecules can separate from one another and enter the gas phase. The strength of the H bond is only about 5% of a typical covalent single bond, but when combined, the overall effect of H bonding can be enormous. H bonding helps to hold two strands of DNA together, but is weak enough to allow the chains to separate for protein synthesis and cell reproduction. Surface Tension 3 In a sample of liquid, intermolecular forces affect the surface of a sample differently than they affect the interior of the liquid. Interior molecules are attracted by others on all sides. Molecules on the surface are only attracted by the molecules on the side and the molecules below it. As a result, the molecules on the surface are pulled inward and downward which causes the surface to behave like a skin. In order for a molecule to be on the surface, or to increase the surface area of the liquid, the molecule must overcome the attractions within the interior of the liquid which requires energy. The stronger the forces are between the particles in a liquid, the greater the surface tension. Water is an amazing little molecule. Oxygen, O2, has a molecular mass of 32 amu or 32 g/mole and we know that O2 is a gas at room temperature. Water, H2O, has a molecular mass of 18 amu or 18 g/mole and it is a liquid at room temperature. We take these simple things for granted. But if O2 was not a gas and H2O was not a liquid at standard temperatures and pressures, WE would NOT be here!! So water is almost half the mass of oxygen – yet it is a liquid. WHY aren’t these two things reversed?? Because of intermolecular forces! These small, very small, attractions between molecules keep our planet – and our life (remember DNA!!) in working order. O2 is a nonpolar molecule – therefore the only intermolecular force that it is subjected to are dispersion forces or other induced dipole forces. This keeps those oxygen molecules AWAY from one another – making oxygen a gas. Water has a dipole moment. It is a polar molecule – it is subjected to London dispersion forces (as are all molecules) but it has that oxygen there with lone pairs and a dipole moment – which means the oxygen is partially negatively charged and the hydrogens are partially positively charged and this makes water an ideal molecule to participate in H bonding. Water has a tetrahedral VSEPR geometry and a bent/angular molecular geometry. This shape and the dipole moment allow for water to engage in 4 hydrogen bonds per 1 molecule of water! That is a LOT of extra attraction/interaction that must be overcome giving rise to water’s unique physical properties. 4 H is represented by the white spheres, O by the red spheres And the lone pairs on O by the purple spheres Water is often called the universal solvent. This is due to its polarity and its ability to H-bond. Water dissolves ionic compounds through ion-dipole interactions that separate the ions in the solid yet keep them in solution. The partially negative O will surround the positive cations while the partially positive H’s will surround the negative anions from the ionic solid. Water can solubilize polar species like alcohols and sugars by hydrogen bonding with them. Water can even solubilize gases through dipoledipole and London dispersion forces. Water has a very high – completely unpredicted – specific heat capacity. Heat capacity is defined as the measure of heat absorbed by a substance to cause a given temperature rise. When energy is put into a system of water, some of that energy causes molecules to vibrate, some to rotate, and some to increase the average kinetic energy or speed of the molecules. It is the increase in the average kinetic energy that we measure by temperature. However, for a given amount of energy put into a system of water, the overall temperature does not rise all that much meaning water stays liquid and does not evaporate into a gas! Remember, all those H bonds have to be broken first – before H2O(l) is converted to H2O(g) meaning that the energy put into the system disrupts the H bonds first. With water covering 70% of our planet and being one of the most important components for life on this planet – it would not be a good thing for the oceans – or our bodies! – if a little input of heat caused water to boil. 5 H bonding also gives rise to the amazingly high surface tension that water has. You can make a pin float on top of water due to its surface tension. It may take some practice – but you can try it. Except for some metals and molten salts, water has the highest surface tension of many liquid. Pretty amazing for such a small molecule! Water also has high capillary action which allows plants to stay hydrated especially during the dryer seasons. When water solidifies, the H bonds become frozen in space. In the liquids, the H bonds are free moving, rotating and flipping and changing as the molecules move and rotate through space. When the temperature drops, the water molecules and the H bonds become locked in a hexagonal form. Snowflakes and ice crystals arise because of this hexagonal open structure of the ice. The large spaces in the ice crystals give rise to the fact that ice actually has a lower density than does liquid water. Ice floats on water. If the solid were denser than the liquid – which is true for nearly every other substance, the surface of lakes would freeze and sink, icebergs would sink, then the surface would freeze again, and then sink, and then the surface would freeze again and sink. Slowly, our water would turn into the solid state for the winter months and aquatic life would not survive. Water density changes slightly as temperature changes and this allows for water to cycle in the system, keeping it all oxygenated and distributing the nutrients. The water cycle (given in the beginning) helps to maintain life on earth as well. Water helps with erosion – which can be both a good and bad thing! – it feeds our plants, and us too! It keeps us cool, and gives us electricity just to name a few things! 6