Problem - NMR Wiki
advertisement
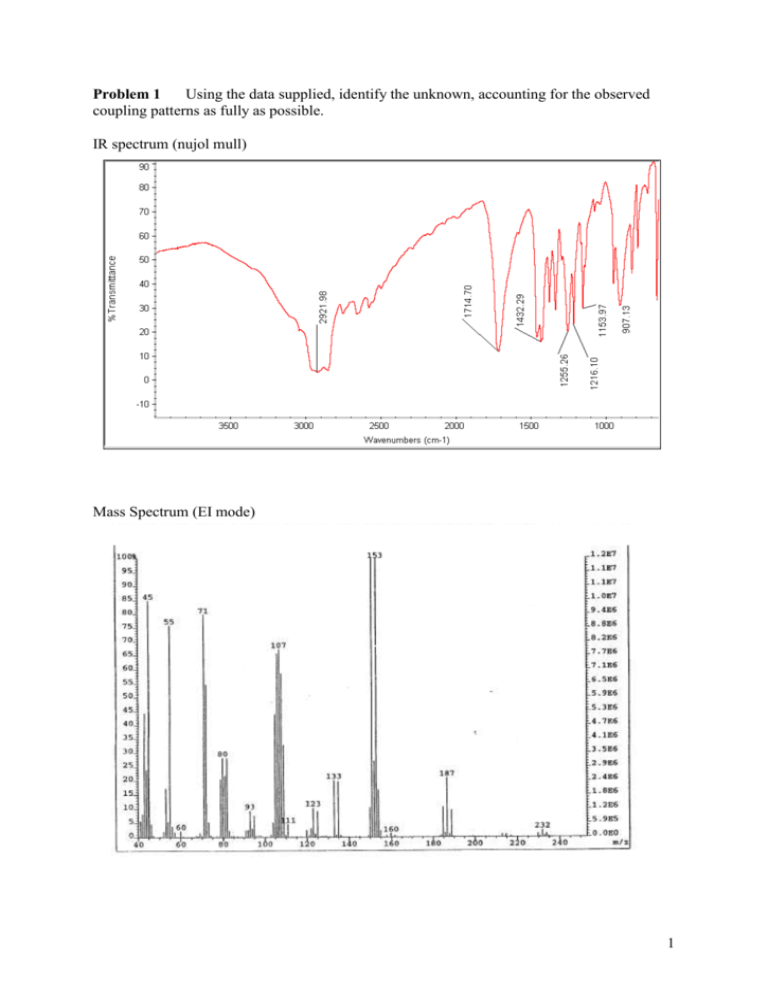
Problem 1 Using the data supplied, identify the unknown, accounting for the observed coupling patterns as fully as possible. IR spectrum (nujol mull) Mass Spectrum (EI mode) 1 1 H NMR spectrum (300 MHz, CDCl3) 4.5 11 10 9 4.2 8 7 25.77 0.94 13 3.9 ppm 6 5 4 22.63 24.50 24.33 3 2 1 ppm 1.83 C NMR spectrum (175 MHz, CDCl3) 2 Problem 2 You need some 2,4-pentane diol as a starting material for a laboratory preparation. After asking around, you track down two bottles of this material. One costs about £1 / ml, the other costs £50 / ml. The IR and CI-MS (Chemical Ionisation Mass Spectrum) of both reagents were identical, and are shown below together with the 1H and 13C NMR spectra of both samples. Account for the differences in the spectra of the two samples (NMR in methanol-d4) and comment upon the coupling patterns in the 1H NMR spectrum of the expensive sample. What explanation can you offer for the difference in cost for the two samples? IR spectrum (neat liquid) Mass Spectrum (CI mode – reagent gas ammonia) MJ MJDIOLC 356 (5.934) Cm (321:356-176:213) 100 Scan CI+ 2.92e5 122 % 105 0 55 60 65 70 75 80 85 90 95 100 105 110 115 120 125 130 135 140 145 m/z 150 3 £50/ml sample 1 H NMR spectrum (500 MHz, CD3OD) 1.65 4.5 4.0 3.5 1.55 3.0 1.45 2.5 ppm 2.0 1.5 ppm 13 C NMR spectrum (125 MHz, CD3OD) 50.0 70 65 60 55 50 45 40 48.5 35 ppm 30 25 ppm 4 £1/ml sample 1 H NMR Spectrum (500 MHz, CD3OD) 1.66 4.5 4.0 13C 3.5 1.60 1.54 3.0 2.5 65 60 ppm 2.0 1.5 ppm NMR spectrum (63 MHz, CD3OD) 50.0 70 1.48 55 50 45 40 48.5 35 ppm 30 25 ppm 5 Problem 3 Given the spectroscopic data attached, determine the structure of the unknown (organic) liquid. You should assign the spectroscopic data fully, and clearly explain your reasoning. IR spectrum (neat liquid) Mass Spectrum (CI mode – reagent gas ammonia) MJ MJCLDIOC 446 (7.434) Cm (413:447-940:1057) 58 100 Scan CI+ 1.99e4 92 57 % 56 74 128 56 122 91 73 105 130 94 76 0 55 60 65 70 75 80 85 90 95 100 105 110 115 120 125 130 135 140 145 m/z 150 6 1 H NMR spectrum (500 MHz, CDCl3) 7.5 7.0 3.95 3.85 6.5 6.0 3.75 5.5 3.65 5.0 4.5 1.06 13 ppm 4.0 3.5 13.88 13.68 40.94 3.0 ppm 30.43 C NMR spectrum (63 MHz, CDCl3) 160 160 140 140 120 120 100 100 80 80 60 60 40 40 20 20 ppm ppm 7 Problem 4 Given the spectroscopic data attached, determine the structure of the unknown (organic) liquid. You should assign the spectroscopic data fully, and clearly explain your reasoning. Mass Spec (EI mode) 91 100 55 41 50 39 121 29 27 172 49 53 51 0 10 1 75 63 65 15 20 30 40 50 60 73 70 79 80 107 90 100 110 129 120 136 130 157 140 150 160 170 H NMR (500 MHz, CDCl3) 3.60 3.54 3.4 3.48 3.0 ppm 2.6 2.23 2.2 4.16 2.19 1.8 ppm 1.4 ppm 3.10 0.98 8 13 C NMR (125 MHz, CDCl3) 60 50 40 30 20 10 ppm IR (neat liquid) 9







