Optimized Structures and the Method of Calculations
advertisement
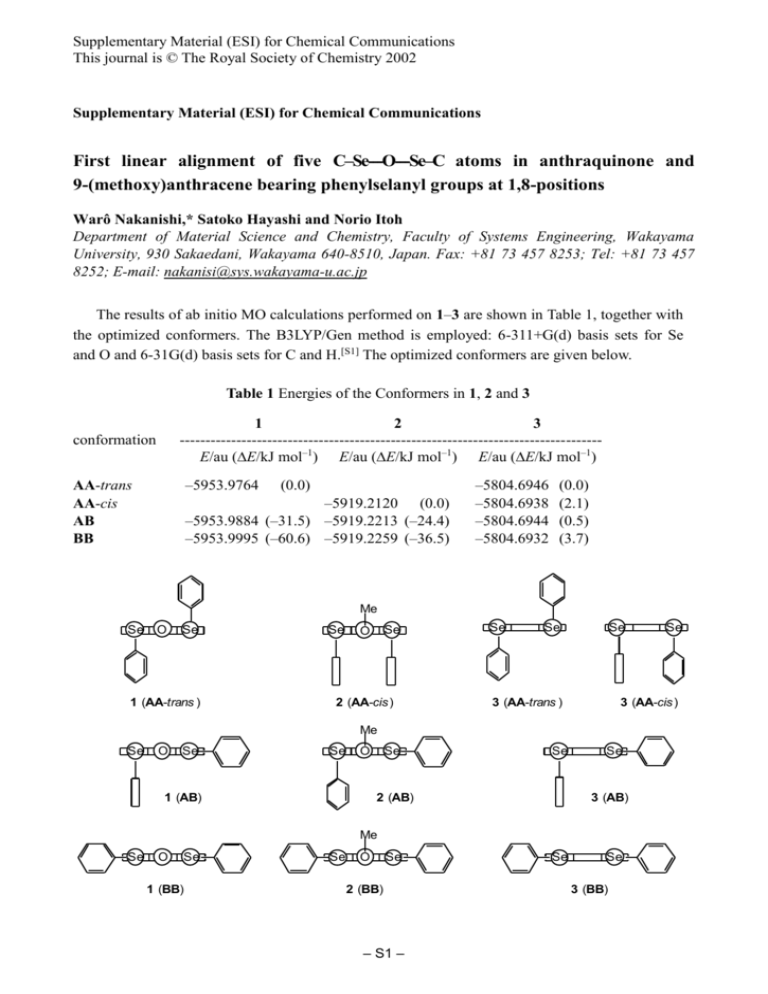
Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Supplementary Material (ESI) for Chemical Communications First linear alignment of five C–Se---O---Se–C atoms in anthraquinone and 9-(methoxy)anthracene bearing phenylselanyl groups at 1,8-positions Warô Nakanishi,* Satoko Hayashi and Norio Itoh Department of Material Science and Chemistry, Faculty of Systems Engineering, Wakayama University, 930 Sakaedani, Wakayama 640-8510, Japan. Fax: +81 73 457 8253; Tel: +81 73 457 8252; E-mail: nakanisi@sys.wakayama-u.ac.jp The results of ab initio MO calculations performed on 1–3 are shown in Table 1, together with the optimized conformers. The B3LYP/Gen method is employed: 6-311+G(d) basis sets for Se and O and 6-31G(d) basis sets for C and H.[S1] The optimized conformers are given below. Table 1 Energies of the Conformers in 1, 2 and 3 1 2 3 ---------------------------------------------------------------------------------E/au (E/kJ mol–1) E/au (E/kJ mol–1) E/au (E/kJ mol–1) conformation AA-trans AA-cis AB BB –5953.9764 (0.0) –5919.2120 (0.0) –5953.9884 (–31.5) –5919.2213 (–24.4) –5953.9995 (–60.6) –5919.2259 (–36.5) –5804.6946 –5804.6938 –5804.6944 –5804.6932 (0.0) (2.1) (0.5) (3.7) Me Se O Se 1 (AA-trans ) Se Se O 2 (AA-cis) Se Se Se 3 (AA-trans ) 3 (AA-cis ) Me Se O Se Se O 1 (AB) Se Se 2 (AB) Se 3 (AB) Me Se O Se 1 (BB) Se O Se 2 (BB) – S1 – Se Se Se 3 (BB) Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 The optimized structures for 1, 2 and 3, are as follows: 1: Optimized conformers, 1 (AA-trans), 1 (AB) and 1 (BB). Conformer: 1 (AA-trans) (type A-type A pairing for the PhSe moieties) Symmetry: C1 E = –5953.9764 au Center Atomic Atomic Coordinates (Angstroms) Number Number Type X Y 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 34 6 6 6 6 6 6 6 8 6 6 6 6 6 6 34 6 6 6 6 6 6 1 1 1 1 1 1 1 1 6 8 1 1 1 6 6 6 6 6 6 1 1 1 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 -2.653348 -2.293992 -3.281369 -3.192577 -2.118345 -1.117345 -1.165107 0.000280 -0.000180 1.166223 1.119259 2.120706 3.194624 3.282665 2.294808 2.653168 2.672301 1.951478 2.030155 2.802554 3.510393 3.459540 4.030960 4.117165 2.853094 1.471657 1.317072 4.143948 3.975799 2.031013 0.001181 0.001567 -2.028047 -3.973404 -4.142879 -2.673450 -1.953389 -2.032776 -2.805122 -3.512199 -3.460639 -4.031470 -4.118926 -2.856212 -1.474864 -1.319009 – S2 – -1.094290 0.816327 1.583960 2.973119 3.622973 2.879322 1.466736 0.707200 -0.510735 1.466105 2.878719 3.621830 2.971395 1.582189 0.815090 -1.095709 -1.579684 -2.704251 -3.127217 -2.415805 -1.284595 -0.872348 -0.007928 -0.729315 -2.740365 -4.003592 -3.229626 1.073410 3.537184 4.702426 3.641074 4.865323 4.703519 3.539331 1.075643 -1.578201 -2.703146 -3.126030 -2.414173 -1.282593 -0.870412 -0.005690 -0.726962 -2.738673 -4.002696 -3.228882 Z 1.208680 1.132001 1.768129 1.846934 1.254445 0.621381 0.567687 -0.000210 -0.000485 -0.567808 -0.621448 -1.254440 -1.846863 -1.768042 -1.132018 -1.208626 0.665715 1.080014 2.409203 3.329004 2.914231 1.581751 1.256505 3.624790 4.364705 2.727163 0.373449 -2.189968 -2.346553 -1.265216 -0.000002 0.000099 1.265250 2.346690 2.190148 -0.665669 -1.080262 -2.409435 -3.328936 -2.913872 -1.581398 -1.255921 -3.624194 -4.364628 -2.727624 -0.373943 Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Conformer: 1 (AB) (type A-type B pairing for the PhSe moieties) Symmetry: C1 E = –5953.9884 au Center Atomic Atomic Coordinates (Angstroms) Number Number Type X Y 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 1 6 1 6 6 6 6 6 6 1 8 34 6 8 6 6 34 6 6 1 1 6 1 6 6 6 6 6 6 6 6 1 1 1 1 1 6 6 6 6 6 1 1 1 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3.318136 2.496404 0.950154 1.186017 1.735088 0.145661 2.766733 0.398421 -1.242498 3.793716 -0.464631 2.143315 -2.353141 -1.472420 -3.634415 -2.121967 -3.223633 -0.717975 -4.710246 -3.756062 -5.708552 -4.506967 -5.351420 -3.234042 4.086661 -2.550450 6.876257 4.686086 4.891022 6.282372 6.077744 4.064001 4.428291 6.900794 6.536620 7.958717 -1.660533 -1.477291 -3.180277 -2.723367 -1.044438 -0.970990 -4.024088 -3.209695 -0.212791 -1.314780 – S3 – 3.919751 3.279582 4.751339 3.749762 1.127572 2.914780 1.988960 1.599688 3.444221 1.645438 -0.426320 -0.667466 2.564276 4.575344 3.122695 1.229751 -1.381609 0.716165 2.366989 4.148878 2.794168 1.043039 0.439808 0.453763 -0.631422 -2.356341 -0.772692 -1.062871 -0.280628 -0.349517 -1.129591 -1.344719 0.044384 -0.075755 -1.463872 -0.828432 -3.910251 -3.236099 -2.252557 -3.019978 -4.021391 -3.286458 -1.581346 -2.932675 -4.706820 -4.514758 Z 1.251146 0.941857 1.332546 0.990773 0.089118 0.586500 0.496438 0.133185 0.636924 0.466531 -0.648660 -0.516987 0.163562 1.044600 0.134336 -0.250234 -1.319871 -0.277762 -0.313041 0.463193 -0.345520 -0.700098 -1.018568 -0.668723 -0.329264 0.212980 -0.127151 0.862123 -1.422177 -1.318410 0.961923 1.707046 -2.349801 -2.169194 1.888711 -0.049136 2.357105 0.037647 1.458597 2.531325 1.109182 -0.920707 1.589127 3.499459 0.969265 3.191241 Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Conformer: 1 (BB) (type B-type B pairing for the PhSe moieties) Symmetry: C2 E = –5953.9995 au Center Atomic Atomic Coordinates (Angstroms) Number Number Type X Y 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 1 6 1 6 6 6 6 6 6 1 8 34 6 8 6 6 34 6 6 1 1 6 1 6 6 6 6 6 6 6 6 1 1 1 1 1 6 6 6 6 6 1 1 1 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0.000534 0.000403 0.000311 0.000285 0.000165 0.000138 0.000343 0.000101 0.000000 0.000420 0.000000 0.000000 -0.000138 0.000000 -0.000285 -0.000101 0.000000 0.000000 -0.000403 -0.000311 -0.000534 -0.000343 -0.000420 -0.000165 -0.000584 0.000584 -0.001486 1.210888 -1.212511 -1.210612 1.208089 2.151130 -2.152405 -2.153569 2.150696 -0.001838 0.001486 1.212511 -1.210888 -1.208089 1.210612 2.152405 -2.151130 -2.150696 2.153569 0.001838 – S4 – 4.625479 3.685658 2.437873 2.475852 2.513900 1.279508 3.705089 1.279786 0.000000 4.658123 0.000000 2.565297 -1.279508 0.000000 -2.475852 -1.279786 -2.565297 0.000000 -3.685658 -2.437873 -4.625479 -3.705089 -4.658123 -2.513900 4.498399 -4.498399 7.240360 5.187466 5.186799 6.555196 6.555863 4.653245 4.652062 7.083702 7.084887 8.304248 -7.240360 -5.186799 -5.187466 -6.555863 -6.555196 -4.652062 -4.653245 -7.084887 -7.083702 -8.304248 Z 3.389540 2.844047 4.619766 3.536133 0.708571 2.821785 1.452300 1.406077 3.588229 0.937058 -0.564425 -1.229672 2.821785 4.812353 3.536133 1.406077 -1.229672 0.671892 2.844047 4.619766 3.389540 1.452300 0.937058 0.708571 -1.493390 -1.493390 -2.061523 -1.641975 -1.641330 -1.921754 -1.922403 -1.538418 -1.537283 -2.034498 -2.035651 -2.282983 -2.061523 -1.641330 -1.641975 -1.922403 -1.921754 -1.537283 -1.538418 -2.035651 -2.034498 -2.282983 Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 2: Optimized conformers, 2 (AA-cis), 2 (AB) and 2 (BB). Conformer: 2 (AA-cis) (type A-type A pairing for the PhSe moieties) Symmetry: E = –5919.2120 au Center Atomic Atomic Coordinates (Angstroms) Number Number Type X Y 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 6 6 6 6 6 6 1 1 1 34 6 6 6 6 6 6 1 1 1 1 1 6 6 6 6 6 6 1 1 1 34 6 6 6 6 6 6 1 1 1 1 1 6 6 1 8 6 1 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1.346032 1.991992 3.181024 3.691731 3.068766 1.886780 1.560532 3.652574 4.583110 -0.262652 -1.656993 -1.464915 -2.537256 -3.804865 -3.992980 -2.921855 -0.487843 -2.379795 -4.637839 -4.973100 -3.069619 1.346032 1.991992 3.181024 3.691731 3.068766 1.886780 1.560532 3.652574 4.583110 -0.262652 -1.656993 -1.464915 -2.537256 -3.804865 -3.992980 -2.921855 -0.487843 -2.379795 -4.637839 -4.973100 -3.069619 1.370029 3.623618 4.522577 0.332754 0.724896 -0.199011 1.308940 1.308940 – S5 – -0.258618 0.182323 0.957767 1.320981 0.898034 0.058212 -0.053259 1.274993 1.939127 -1.285428 -0.030189 1.355233 2.207590 1.689007 0.305943 -0.555089 1.768015 3.283193 2.357365 -0.109278 -1.631067 -0.258618 0.182323 0.957767 1.320981 0.898034 0.058212 -0.053259 1.274993 1.939127 -1.285428 -0.030189 1.355233 2.207590 1.689007 0.305943 -0.555089 1.768015 3.283193 2.357365 -0.109278 -1.631067 -0.385659 1.277779 1.889604 -1.272571 -2.647477 -3.226845 -2.886726 -2.886726 Z 2.559405 3.696814 3.647799 2.435754 1.221874 1.248186 4.664078 4.573420 2.364728 2.960933 2.481961 2.507111 2.238788 1.966813 1.946753 2.190405 2.738254 2.253594 1.767303 1.726576 2.146764 -2.559405 -3.696814 -3.647799 -2.435754 -1.221874 -1.248186 -4.664078 -4.573420 -2.364728 -2.960933 -2.481961 -2.507111 -2.238788 -1.966813 -1.946753 -2.190405 -2.738254 -2.253594 -1.767303 -1.726576 -2.146764 0.000000 0.000000 0.000000 0.000000 0.000000 0.000000 0.896884 -0.896884 Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Conformer: 2 (AB) (type A-type B pairing for the PhSe moieties) Symmetry: C1 E = –5919.2213 au Center Atomic Atomic Coordinates (Angstroms) Number Number Type X Y 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 1 6 1 6 6 6 6 6 6 1 8 34 6 6 6 34 6 6 1 1 6 1 6 6 6 6 6 6 6 6 1 1 1 1 1 6 6 6 6 6 1 1 1 1 1 1 6 1 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3.223101 2.396944 0.872686 1.104854 1.680929 0.027225 2.681605 0.300047 -1.296324 3.716801 -0.506240 2.130895 -2.378207 -3.711290 -2.145841 -3.306729 -0.797255 -4.784012 -3.847241 -5.797429 -4.576226 -5.438869 -3.313760 4.024126 -2.469094 6.751593 4.436383 4.984609 6.344964 5.796443 3.692312 4.666702 7.084822 6.109473 7.809739 -1.314682 -1.305321 -3.056674 -2.470806 -0.736954 -0.835611 -3.965400 -2.925501 0.166317 -0.866324 -1.491886 -0.572203 -1.578007 0.163954 -0.337784 – S6 – 3.930190 3.328118 4.666501 3.737827 1.286528 2.945709 2.096498 1.707901 3.366194 1.787789 -0.066263 -0.466977 2.606259 3.089179 1.343031 -1.274673 0.994722 2.359919 4.051778 2.728766 1.072962 0.459517 0.557561 -0.536558 -2.180773 -0.809836 -1.004598 -0.212217 -0.349495 -1.136334 -1.263190 0.146809 -0.097015 -1.498830 -0.917223 -3.596646 -2.926140 -2.141834 -2.840094 -3.642163 -2.924768 -1.568842 -2.803109 -4.222182 -4.147242 4.319709 0.246535 0.581789 1.019584 -0.677341 Z 1.528703 1.159882 1.849417 1.335072 0.023638 0.838302 0.512731 0.139546 0.986750 0.427998 -1.221455 -0.713874 0.536989 0.713428 -0.137937 -1.158033 -0.396595 0.285391 1.199917 0.416309 -0.280486 -0.520968 -0.484299 -0.274348 0.341219 0.301380 0.981449 -1.241549 -0.952413 1.267599 1.729319 -2.216184 -1.707531 2.243319 0.524674 2.455981 0.128669 1.611595 2.668097 1.186295 -0.849056 1.771084 3.654745 1.016662 3.278676 1.471844 -2.620404 -2.894854 -2.872401 -3.151069 Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Conformer: 2 (BB) (type A-type A pairing for the PhSe moieties) Symmetry: Cs E = –5919.2259 au Center Atomic Atomic Coordinates (Angstroms) Number Number Type X Y 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 6 6 6 6 6 6 1 1 1 34 6 6 6 6 6 6 1 1 1 1 1 6 6 6 6 6 6 1 1 1 34 6 6 6 6 6 6 1 1 1 1 1 6 6 1 8 6 1 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0.866506 1.606835 3.018193 3.682393 2.969279 1.538814 1.110834 3.557606 4.757184 -1.087289 -1.337031 -1.531820 -1.795696 -1.868178 -1.678173 -1.415973 -1.476026 -1.946407 -2.075823 -1.738089 -1.272590 0.866506 1.606835 3.018193 3.682393 2.969279 1.538814 1.110834 3.557606 4.757184 -1.087289 -1.337031 -1.531820 -1.795696 -1.868178 -1.678173 -1.415973 -1.476026 -1.946407 -2.075823 -1.738089 -1.272590 0.899458 3.636130 4.714817 -0.414478 -0.557342 -1.630195 -0.098909 -0.098909 – S7 – -0.122246 -0.011831 0.148484 0.231219 0.114868 -0.111437 -0.017279 0.229661 0.387683 -0.148665 0.161962 -0.919416 -0.691606 0.614235 1.694660 1.471761 -1.934273 -1.534530 0.790179 2.712355 2.310030 -0.122246 -0.011831 0.148484 0.231219 0.114868 -0.111437 -0.017279 0.229661 0.387683 -0.148665 0.161962 -0.919416 -0.691606 0.614235 1.694660 1.471761 -1.934273 -1.534530 0.790179 2.712355 2.310030 -0.307243 0.194632 0.333688 -0.732734 -2.165812 -2.362618 -2.592445 -2.592445 Z 2.524574 3.680065 3.648422 2.456485 1.225771 1.242774 4.643561 4.588384 2.420649 2.616109 4.517883 5.387392 6.740384 7.229576 6.364407 5.011178 5.004464 7.409764 8.281707 6.741245 4.335703 -2.524574 -3.680065 -3.648422 -2.456485 -1.225771 -1.242774 -4.643561 -4.588384 -2.420649 -2.616109 -4.517883 -5.387392 -6.740384 -7.229576 -6.364407 -5.011178 -5.004464 -7.409764 -8.281707 -6.741245 -4.335703 0.000000 0.000000 0.000000 0.000000 0.000000 0.000000 0.898957 -0.898957 Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 3: Optimized conformers, 3 (AA-trans), 3 (AA-cis), 3 (AB) and 3 (BB). Conformer: 3 (AA-trans) (type A-type A pairing for the PhSe moieties) Symmetry: C1 E = –5804.6946 au Center Atomic Atomic Coordinates (Angstroms) Number Number Type X Y 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 6 6 6 6 6 6 6 6 6 6 6 6 6 6 1 1 1 1 1 1 1 1 34 34 6 6 6 6 6 6 1 1 1 1 1 6 6 6 6 6 6 1 1 1 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3.166479 2.170026 1.061192 1.056364 2.114765 3.136443 0.001737 0.007373 -1.044423 -1.055072 -2.166963 -3.161006 -3.125151 -2.100234 -0.000335 3.995970 2.088739 3.939281 0.009561 -3.993051 -3.926105 -2.069698 2.282025 -2.286569 2.676129 2.179607 2.492974 3.281618 3.766558 3.476925 1.541885 2.105965 3.514761 4.382661 3.869404 -2.681654 -3.457229 -3.747756 -3.288654 -2.525094 -2.211116 -3.829841 -4.344162 -3.522222 -2.158254 -1.593090 – S8 – 1.650249 0.924471 1.595662 3.042667 3.749531 3.074538 0.921158 3.721326 3.046239 1.599274 0.931982 1.661183 3.085296 3.756717 -0.161423 1.130828 4.836529 3.614991 4.809461 1.144631 3.628511 4.843597 -1.008590 -1.000506 -1.453466 -2.661004 -3.051334 -2.232182 -1.023133 -0.635787 -3.289199 -3.991207 -2.532048 -0.380110 0.297850 -1.444631 -0.612358 -0.999893 -2.222759 -3.056156 -2.666543 0.332522 -0.345545 -2.522451 -4.007098 -3.306877 Z 1.869198 1.258017 0.621129 0.628359 1.278484 1.888478 -0.000258 -0.001678 -0.630910 -0.622233 -1.257912 -1.868979 -1.889815 -1.281281 0.000338 2.338813 1.278022 2.382045 -0.002247 -2.337222 -2.383400 -1.282064 1.373152 -1.372193 -0.476972 -0.981437 -2.285634 -3.095473 -2.590611 -1.281409 -0.365246 -2.670674 -4.113349 -3.214010 -0.889830 0.477766 1.291755 2.600770 3.096189 2.276778 0.972625 0.907859 3.231495 4.114011 2.654320 0.348832 Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Conformer: 3 (AA-cis) (type A-type A pairing for the PhSe moieties) Symmetry: C1 E = –5804.6938 au Center Atomic Coordinates (Angstroms) Number Number X Y Z 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 1 6 1 6 6 6 6 6 6 1 1 34 6 1 6 6 6 6 1 1 6 1 6 6 6 6 6 6 6 1 1 1 1 1 34 6 6 6 6 6 6 1 1 1 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 -5.967125 -4.916591 -4.549755 -4.134311 -3.032248 -2.753241 -4.357139 -2.175834 -1.944853 -4.988973 -0.396570 -2.399281 -0.596947 -2.379380 0.212257 -0.018503 -0.827770 1.525100 -0.240196 2.135655 2.102917 3.141533 1.368472 -1.295335 -1.829287 -1.028708 0.296037 0.822577 0.031456 -2.861981 -1.444902 0.915639 1.856898 0.451142 2.241030 3.100958 4.059492 4.704336 4.405342 3.450929 2.794103 4.310588 5.450287 4.913951 3.206924 2.047540 – S9 – 0.908705 0.945154 2.819988 2.000744 -0.126039 2.055174 -0.128413 0.980339 3.134706 -0.966120 0.271512 -1.617872 3.217438 3.939872 4.328928 2.153958 1.066385 4.407591 5.115002 5.256742 3.377742 3.460987 2.280505 -2.528517 -2.921392 -3.606542 -3.918402 -3.536489 -2.835711 -2.690096 -3.905428 -4.454405 -3.758434 -2.515827 0.966315 -0.161392 -1.068411 -1.926791 -1.882184 -0.975016 -0.117078 -1.100871 -2.623369 -2.545618 -0.933636 0.576225 1.027675 0.753038 1.715101 1.133357 -0.368125 0.769258 0.001096 -0.009638 1.140680 -0.277179 -0.976755 -1.442062 0.772742 1.729656 1.159552 -0.021720 -0.381136 0.780243 1.759305 1.073955 -0.014791 -0.318710 -0.401935 -0.123648 1.109165 2.023904 1.706017 0.471520 -0.443517 1.352758 2.982650 2.419824 0.224793 -1.392679 -1.534774 -0.208534 -0.679933 0.211753 1.575894 2.039630 1.154624 -1.737665 -0.162588 2.269821 3.098089 1.527407 Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Conformer: 3 (AB) (type A-type B pairing for the PhSe moieties) Symmetry: C1 E = –5804.6944 au Center Atomic Atomic Coordinates (Angstroms) Number Number Type X Y 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 6 6 6 6 6 6 6 6 6 6 6 6 6 6 1 1 1 1 1 1 1 1 34 34 6 6 6 6 6 6 1 1 1 1 1 6 6 6 6 6 6 1 1 1 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 4.497326 3.228279 2.109113 2.373778 3.709733 4.741345 0.789938 1.308394 -0.008389 -0.277724 -1.640467 -2.643211 -2.362368 -1.084147 0.601353 5.329744 3.887442 5.755621 1.510123 -3.666302 -3.182613 -0.865818 3.012242 -1.986276 2.507338 1.593402 1.252651 1.803010 2.707001 3.070351 1.140206 0.544799 1.528237 3.140808 3.783975 -3.920828 -4.578430 -5.971971 -6.710927 -6.055496 -4.661143 -4.000554 -6.479219 -7.795308 -6.626897 -4.147768 – S10 – 1.135045 0.603244 1.385147 2.727695 3.234499 2.464319 0.914484 3.508072 3.035068 1.691338 1.219134 2.036054 3.360615 3.848746 -0.086933 0.531216 4.247685 2.853033 4.520077 1.678291 3.978064 4.857311 -1.175954 -0.609376 -2.173914 -3.224209 -4.006645 -3.731525 -2.675497 -1.902917 -3.424042 -4.822734 -4.333994 -2.455728 -1.092247 -0.593044 -0.121277 -0.167498 -0.690456 -1.165744 -1.117531 0.278253 0.200104 -0.729438 -1.574052 -1.482869 Z -0.721790 -0.715788 -0.246248 0.222567 0.191991 -0.271199 -0.219386 0.685190 0.715621 0.252135 0.298859 0.761245 1.200497 1.184748 -0.588374 -1.069541 0.544408 -0.291808 1.030620 0.802137 1.556972 1.526154 -1.457447 -0.273469 0.131706 -0.013358 1.093148 2.346169 2.487517 1.383541 -0.980655 0.973879 3.207524 3.459690 1.496200 -0.346711 -1.490555 -1.559412 -0.495052 0.642309 0.720154 -2.318293 -2.447440 -0.553779 1.471659 1.604403 Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Conformer: 3 (BB) (type B-type B pairing for the PhSe moieties) Symmetry: C2 E = –5804.6932 au Center Atomic Atomic Coordinates (Angstroms) Number Number Type X Y 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 6 6 1 1 6 6 6 6 6 6 1 1 1 6 6 6 6 6 6 1 1 1 34 6 6 6 6 6 6 1 1 1 1 1 34 6 6 6 6 6 6 1 1 1 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0.000000 0.000000 0.000000 0.000000 -0.021655 -0.019028 -0.007423 -0.002901 0.000000 -0.007801 -0.000957 -0.024942 -0.029704 0.021655 0.019028 0.007423 0.002901 0.000000 0.007801 0.000957 0.024942 0.029704 0.037077 -0.220407 -1.515446 -1.696655 0.888158 0.699301 -0.591045 -2.702209 -2.372027 1.561383 1.889754 -0.735607 -0.037077 0.220407 -0.888158 -0.699301 1.515446 1.696655 0.591045 -1.561383 -1.889754 2.702209 2.372027 0.735607 – S11 – 0.000000 0.000000 0.000000 0.000000 3.653005 2.468233 1.227215 1.230328 2.504818 3.673631 4.631403 2.449682 4.598216 -3.653005 -2.468233 -1.227215 -1.230328 -2.504818 -3.673631 -4.631403 -2.449682 -4.598216 2.493891 4.376194 4.903665 6.249807 5.196087 6.541340 7.068499 6.656660 4.262734 7.174995 4.782334 8.114796 -2.493891 -4.376194 -5.196087 -6.541340 -4.903665 -6.249807 -7.068499 -7.174995 -4.782334 -6.656660 -4.262734 -8.114796 Z 0.894047 3.684712 4.772922 -0.191209 3.034352 3.718847 3.012061 1.565833 0.890078 1.610790 1.101989 4.805605 3.570683 3.034352 3.718847 3.012061 1.565833 0.890078 1.610790 1.101989 4.805605 3.570683 -1.057265 -1.427183 -1.514093 -1.837297 -1.672609 -1.999153 -2.080610 -1.903275 -1.329355 -2.189642 -1.606203 -2.336017 -1.057265 -1.427183 -1.672609 -1.999153 -1.514093 -1.837297 -2.080610 -2.189642 -1.606203 -1.903275 -1.329355 -2.336017 Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Experimental Section Chemicals were used without further purification unless otherwise noted. Solvents were purified by standard methods. 1H NMR spectra were measured at 300 MHz. The 1H chemical shifts are given in ppm relative to those of internal TMS in CDCl3 solvent. Column chromatography was performed on silica gel that covered with basic alumina layer on the top. 1,8-Bis(phenylselanyl)anthraquinone (1). To 3.00 g (9.61 mmol) of diphenyl diselenide in 40 ml of ethanol, was added 1.10 g (29.10 mmol) of NaBH4 under Ar atmosphere. The solution was added 40 ml of benzene and distilled until solution was ca 25 ml. Then 80 ml of DMF was added to the solution and distilled until temperature of solution was 110 °C. After the solution was cooled to 45 °C, 2.40 g (8.66 mmol) of 1,8-dichloroanthraquionone and 4.30 g (22.58 mmol) of CuI were added to the solution. The mixture was refluxed over 20 hrs and cooled to room temperature. After usual work-up, the crude product was chromatographed on silica gel that covered with basic alumina layer on the top and recrystallized from ethanol–chloroform. 1 gave 78 % yield as dark red prisms: mp 199.0 °C (DSC), 1H NMR 7.30 (d, J = 8.1 Hz, 2H), 7.41 (t, J = 7.8 Hz, 2H), 7.42–7.52 (m, 6H), 7.73–7.80 (m, 4H), 8.11 (d, J = 7.5 Hz, 2H). Anal. Calcd for C26H16O2Se2: C, 60.25; H, 3.11. Found: C, 60.32; H, 3.15. 1,8-Bis(phenylselanyl)-9-(methoxyl)anthracene (2). To a solution of 2.00 g (5.45mmol) of 1,8-dibromo-9-methoxyanthracene (6) in 30 ml of dry THF at –78 °C was added dropwise 7 ml of n-BuLi (1.6 M in hexane) via syringe, and the mixture was stirred at the same temperature for 2 hrs. Freshly prepared 2.84 g (12.02 mmol) of phenylselanylbromide in 10 ml of dry THF was added to the orange suspension at –78 °C dropwise using canuula. The reaction mixture was maintained with stirring at –78 °C for 1.2 hrs, and then temperature of the one was raised to 65 °C slowly for 1 hr. After usual work-up, the crude product was chromatographed on silica gel with benzene: hexane = 1:1–4:1 eluent and recrystallization from ethanol–chloroform. 2 gave 41 % yield as yellow needle: mp 221.6 °C (DSC), 1H NMR (CDCl3) 4.03 (s, 3H), 6.88 (dd, J = 0.7 and 7.3 Hz, 2H), 7.11 (t, J = 7.7 Hz, 2H), 7.41–7.50 (m, 6H), 7.70 (d, J = 8.3 Hz, 2H), 7.77–7.84 (m, 4H), 8.18 (s, 1H). Anal. Calcd for C27H20OSe2: C, 62.56; H, 3.89. Found: C, 62.61; H, 3.88. 1,8-Bis(phenylselanyl)anthracene (3).[S2] To 2.00 g (3.86 mmol) of 1 in 60 ml of 1,4-Dioxane, added 2.52 g (38.6 mmol) of Zinc powder and 0.096 g (0.39 mmol) of CuSO4•5H2O and then added 120 ml of 28 % NH3aq, slowly. The solution was heated to reflux over 4 hrs. The solvent was removed in vacuo. The residue was dissolved in 80 ml of ethanol and 1 ml of 36 % HClaq. The solution was heated to reflux during 1 hr. The reaction mixture was concentrated in vacuo. The crude product was chromatographed on silica gel and recrystallized from ethanol. 3 gave 86 % yield as bright yellow prisms: mp 126.8 °C (DSC), 1H NMR 7.13–7.24 (m, 6H), 7.38–7.49 (m, 4H), 7.35 (t, J = 7.6 Hz, 2H), 7.76 (dd, J = 1.1 and 6.8 Hz, 2H), 7.97 (d, J = 8.5 Hz, 2H), 8.42 (s, 1H), – S12 – Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 9.48 (s, 1H). Anal. Calcd for C26H18Se2: C, 63.95; H, 3.72. Found: C, 63.89; H, 3.64. 1,8-Dibromo-9-methoxyanthracene (6).[S2] To a solution of 77.74 g (1943.61 mmol) of NaOH and 3.82 g (11.84 mmol) of (n-Bu)4NBr in 250 ml of H2O, was added, dropwise and with vigorously stirring during 45min, a solution of 13.29 g (37.74 mmol) of 1,8-dibromo-9-anthrone (7) and 19.92 g (157.92 mmol) of dimethyl sulfate in 215 ml of CH2Cl2. The resulting mixture was stirred at 25 °C for additional 1.5 hrs. After usual work-up, the crude product was recrystallized from chloroform–acetone to give 6.45 g of 6 as orange prisms (47 % yield): mp 178.6 °C (DSC), 1H NMR (CDCl3)3.86 (s, 3H), 7.24 (t, J = 7.7 Hz, 2H), 7.85 (d, J = 7.2 Hz, 2H), 7.91 (d, J = 8.3 Hz, 2H), 8.23 (s, 1H). 1,8-Dibromo-9-anthrone (7).[S2] 17.00 g (46.45 mmol) of 1,8-dibromoanthraquinone (8) was dissolved in 155 ml of concentrated sulfuric acid with vigorously stirring. Then 4.01 g (148.63 mmol) of Al powder was added and the mixture was stirred for 42 hrs, while the temperature of the mixture was maintained at 30–40 °C. The resulting yellow suspension was poured onto 500 ml of ice water and after standing for 20 min, was filtered. After usual work-up, the crude product was recrystallized from CH2Cl2–ethanol to give 13.54 g of 7 as tan needles (83 % yield): 1H NMR (CDCl3) 4.17 (s, 2H), 7.29 (d, J = 7.7 Hz, 2H), 7.34 (t, J = 7.7 Hz, 2H), 7.64 (d, J = 7.7 Hz, 2H). 1,8-Dibromoanthraquinone (8).[S3] To a suspension of 10.00 g (36.09 mmol) of 1,8-dichloroanthraquinone, 21.47 g (180.44 mmol) of KBr, and 0.43 g (2.53 mmol) of CuCl2・2H2O in 87 ml of nitrobenzene, was added 11 ml of 85 % H3PO4 with vigorously stirring, and then refluxed for 42 hrs. After one-thirds of the solution was steam distilled, cooling and filtration, the residue was recrystallized from chloroform–ethanol to give 7.81 g of 8 as yellow prisms (59 % yield): mp 235.9 °C (DSC), 1H NMR (CDCl3) 7.55 (t, J = 7.9 Hz, 2H), 8.03 (d, J = 7.9 Hz, 2H), 8.24 (dd, J = 1.3 and 7.7 Hz, 2H). Reference [S1] Gaussian 98, Revision A.9; Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery, Jr., J. A.; Stratmann, R. E.; Burant, J. C.; Dapprich, S.; Millam, J. M.; Daniels, A. D.; Kudin, K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G. A.; Ayala, P. Y.; Cui, Q.; Morokuma, K.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz, J. V.; Baboul, A. G.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Andres, J. L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E. S.; Pople, J. A. Gaussian, Inc., Pittsburgh PA, 1998. [S2] H. O. House, J. A. Hrabie, D. VanDerveer, J. Org. Chem., 1986, 51, 921. – S13 – Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 [S3] I. G. Farbenindustrie, D.R.P. 1931, 597259; Frdl. 21, 1112. – S14 –