Supplementary Information (doc 28K)
advertisement

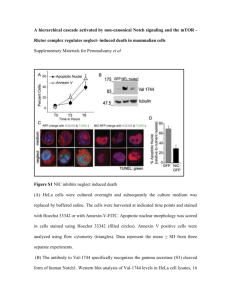
Supplemental procedure Construction of plasmid p3XFLAG-CMV14-hASCT2 Total RNA of HeLa S3 cells was extracted by Isogen II (Nippon Gene; Japan) following the manufacturer’s instruction. First strand cDNA of HeLa S3 cells was synthesized from total RNA by using SuperScript III first-strand synthesis system with oligo dT primer (Invitrogen; CA, USA). Coding sequences of ASCT2 (CCDS12692.1) was amplified from HeLa S3 cDNA template CTCTTTCAGGGACCCATGGTGGCCGATCCTCCT-3’ using and ACCGCGTGGCACCAGCATGACTGATTCCTTCTCAGAG-3’. forward primer 5’- reverse primer 5’- reaction was PCR conducted by using KOD FX DNA polymerase (Toyobo; Japan). cDNA of ASCT2 was cloned into pTriEx-6 vector (Novagen; Germany) by In-fusion kit (Clontech; Mountain View) to obtained pTriEx6-hASCT2. The ASCT2 from pTriEx6-hASCT2 was then amplified by using forward primer 5’-ATAAAGCTTATGGTGGCCGATCC-3’ and reverse primer 5’-CCCTCTAGACATGACTGATTCCTTC-3’. PCR reaction was conducted by using PrimeSTAR Max DNA polymerase (Takara; Japan). The ASCT2 was subcloned into p3XFLAG-CMV-14 (Sigma-Aldrich; MO, USA) at HindIII and XbaI restriction sites to obtain p3XFLAG-CMV14-hASCT2. DNA transfection, membrane extraction, western blot and ASCT2 detection HEK 293T (ATCC) was cultured in Dulbecco’s Modified Eagle Medium (Wako; Japan) with supplement of 10% fetal bovine serum (Gibco; NY, USA) at 37oC, 5% CO2 and humidity. The cells were transfected with p3XFLAG-CMV14-hASCT2 or p3XFLAGCMV14 (for mock cells) by using Lipofectamine LTX with Plus reagent (Invitrogen; CA, USA) and further cultured for 48 hours. Transfected cells were collected and the crude membrane fraction was obtained as described (Khunweeraphong et al). Equal amount of the membrane fraction from the cells transfected with ASCT2 and mock cells were dissolved in suspension buffer containing 1% Fos-Choline 12 (Affymetrix; CA, USA) and incubate on ice for 10 min. Membrane samples were then mixed with the Laemmli sample buffer containing 100 mM DTT and subjected to SDS-PAGE. After SDSPAGE, separated proteins were transferred electrophoretically to Hybond P PVDF membrane (GE Healthcare Life Science; PA, USA). The membrane was blocked for 1 h at room temperature with TBS-T (10 mM Tris pH 7.6 + 150 mM NaCl + 0.1% Tween 20) containing 5% (w/v) skim milk. The membrane was then incubated for 2 h at room temperature with TBS-T containing 5% (w/v) skim milk and 1:5,000 dilution of the primary antibodies; either anti-ASCT2 polyclonal antibody produced in rabbit or anti-FLAG polyclonal antibody produced in rabbit (Sigma-Aldrich; MO, USA). The membrane was washed with TBS-T and subsequently incubated for 1 h at room temperature with TBS-T containing 5% (w/v) skim milk and 1:5,000 dilution of horseradish-peroxydase-conjugated anti rabbit IgG (Jackson ImmunoResearch Laboratory; PA, USA). The membrane was washed again with TBS-T. The signals on membrane were developed by SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific; IL, USA) and visualized under the LAS-4000 mini Luminescent image analyzer (Fujifilm; Japan). Reference: Khunweeraphong N, Nagamori S, Wiriyasermkul P, Nishinaka Y, Wongthai P, Ohgaki R, Tanaka H, Tominaga H, Sakurai H, Kanai Y. J Pharmacol Sci. 2012;119(4):368-80. Epub 2012 Jul 31. Figure legend for supplement Figure 1 Western blots were performed on HEK 293Tcells transfected human 3FLAGASCT2 as described in Supplemental procedure. In the western blots, the polyclonal antiASCT2 antibody recognized a band that is also detected with anti-FLAG antibody for the transfected cells, confirming the specificity of the anti-ASCT2 antibody used in this study.