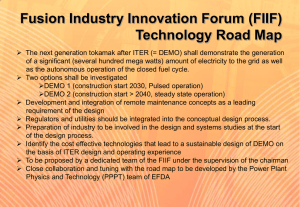
Physics 104 - Root page for umdgrb.umd.edu
advertisement

Physics 104 - How Things Work. Greg Sullivan Fall, 2000 Introduction of who I am. Professor here Research in Particle Astrophysics o Elementary particles & fields Purpose of course: How things work o Scientific bases of how things work o From outside to inside and the underlying scientific principles Grading: Homework 15% 2 - 1 Hr exams 20% each Term paper 20% Final exam 25% o Comprehensive Homework: Practice o OK to help each other but…. Term papers: Look at the web page Exams: Closed book ~75 minutes Stress concepts o Some short answer o Some numerical 1 Don’t want you to remember a bunch of formulas (some). I want you to know how things work not remember ho they work. Ask Questions Cliché – The only stupid question is the one you don’t ask. TA: Yung-Fu Chen yfuchen@physics.umd.edu physics 1322 Book: How Things Work – The Physics of Everyday Life o Louis A. Bloomfield Hand out the syllabus, policy, & schedule. Physics. What is Physics?? Try to break down the complexity of the world into simple rules. A few basic laws o Periodic table o Example of the soccer game Watch the game and try to figure out the rules from what happens on the field DEMO – C3-02: Table Cloth trick Why do the dishes stay there when I pull out the table cloth? o What are the rules? o Do they apply everywhere? 2 Issue: Language o Often physics terms have a meaning different then in normal usage. (e.g. conservation of energy) So, in order to set a foundation for the entire course, and for your basic understanding, we will start with The Laws of Motion Begin Section 1.1 DEMO – Throw tennis balls around room Falling Balls What are the rules? Are there rules? Questions: Does tennis ball & baseball follow the same rules? Under what conditions do they have the same path? Horizontal motion vs. falling motion? How does weight affect the motion? History: Aristotle 350BC o VF Heavier fall faster Galileo 1600 o All fall equal (tower of Pisa) 3 DEMO - C4-33: FREE FALL IN VACUUM - FEATHER AND BALL - C4-34: GALILEO'S EXPERIMENT - MASSES IN FREE FALL The motion of an isolated ball No gravity Correct answer eluded people for thousands of years Galileo’s law of inertia Inertia: A Body in motion tends to remain in motion; a body at rest tends to remain at rest. Falling ball more complicated Accurate description need several physical quantities o Position o Speed o Velocity o Mass o Acceleration o Force Location of ball: Position o Distance & direction from reference point 3 spatial coords wrt a reference 4 DEMO - A2-01: CARTESIAN COORDINATE AXES o e.g. 30 miles north of Campus o 10 miles east of my house could both be same location! Position is an example of a vector quantity. Both magnitude and direction If a ball is moving, then its position is changing. Velocity o How quickly the position is changing o Speed the ball is moving & direction it is heading. speed distance time o Velocity is vector quantity with speed & direction 50mph due north Newton - ~1660 Armed with this terminology: Inertia stated as Newton’s 1st Law: An object that is not subject to any outside force moves at a constant velocity, covering equal distances in equal times (speed) along a straight line path(direction). 5 DEMO - C4-04: F=MA WITH ULI AND FORCE PROBE use demo to show graph of constant velocity motion Why is dV/dt = 0? Because of mass o Inertial Mass What takes more force? o To stop a heavy or a light object? DEMO - C3-04: INERTIA - LEAD BRICK AND HAND C3-12: PENCIL AND PLYWOOD 6 OK Back to falling balls again DEMO - C2-06: BALL DROP ON ROPE - EQUAL AND UNEQUAL INTERVALS What happens? Are equal distances covered in equal times? What does the 1st Law tell us? o There must be an out side force! Gravity pulling down! DEMO - C2-07: FREE FALL - EQUAL TIME INTERVALS When something pushes on the ball, its velocity changes It accelerates 7 Acceleration: Change in velocity per unit time acceleration change in velocity time Vector quantity like position & velocity o Magnitude & direction Direction along the direction of push (force) DEMO - C4-04: F=MA WITH ULI AND FORCE PROBE show position vs velocity vs acceleration graphs What objects are accelerating? 1. car going straight down the road 2. constant speed on the beltway 3. object in orbit (satellite) 4. putting breaks on at 55mph 5. falling ball 6. thrown baseball If we push an object its velocity changes, that is it accelerates Direction of the change in velocity is along the pusj. 8 A Simple rule that relates the push (force), the acceleration, and the inertia (mass). Newton’s 2nd Law The force exerted on an object is equal to the product of that object’s mass times its acceleration. The acceleration is in the same direction as the force. F ma or by rearranging the formula: F a m acceleration is proportional to Force divided by the inertia(mass) and is along the direction of the force. DEMO - C4-02: AIR TRACK - A = F/M (with ULI) NOTE: at start of 2nd lecture use computer with internet connection displayed on projector to access the phys 104 class website http://umdgrb.umd.edu/sullivan/physics104.html and to join hypernews. 9 Units: SI Units (metric) length (distance) meters , m time seconds, s mass kilograms. Kg So, for example velocity = m/s acceleration = m/s/s or m/s2 Force – kg m/s2 = 1 Newton ,N o 1 N ~ force created by 10 US quarters in your hand Weight & Gravity: Why does an object weight something? Gravity Pull of gravity from earth o Moon & sun too far away More subtle – ocean tides Remarkable thing about gravity o Weight is proportional to mass w mg Where g is a constant that is determined by the local strength of gravity. The constant g is called the acceleration due to gravity. Determined by the properties of Earth. o Radius, mass o Doesn’t depend on the object being considered g = 9.8 m/s2 (32 ft/s2) on earth’s surface Objects will weigh something different on another planet or moon On moon weight = 1/6 that of earth gmoon = 1/6 gearth 10 Back again to the Falling Balls: Only force on the falling ball is its weight. How much will it accelerate? minertial a F w mgravity g minertial a mgravity g ag The acceleration of the object due to the force of gravity is independent of the mass of the object. Remember the demo with the feather & ball Newton & Einstein got famous for this Consider a baseball and a bowling ball: Although the bowling ball weighs more (has more force due to gravity), it also has more mass (inertia) which resists the force more. So, the greater inertia exactly cancels the greater force and they both end up with the same acceleration, and therefore the same motion. Now we can examine the motion of any falling ball near the earth’s surface. Any ball will accelerate downward at a constant rate of 9.8 m/s2. What about velocity & position of falling ball? (1D) Velocity V = V0 + a t Example: throw a ball up at 20 m/s (~45mph). V = 20m/s –9.8m/s2 x t When does it stop? V=0 = 20 –9.8 x t t ~ 2s What does this mean? What is a at this point? 11 What about position? Distance = <vel> x time <vel> = ½(V0 + Vf) = ½ (V0 + (V0 + at)) <vel> = initial velocity + ½ acceleration x time present position = initial position + Distance X = X0 + V0 * t + ½ * a * t2 Second order in time Parabola Summarize falling ball: (draw this geometrically as in book) T(s) 0 1 2 3 A(m/s2) -9.8 -9.8 -9.8 -9.8 V(m/s) 0 -9.8 -19.6 -29.4 P(m) 0 -4.9 -19.6 -44.1 Recall V & X graphs from Air-track demo with ULI falling Ball 0 0 -5 -5 -10 -15 Position(m) Velocity (m/s) -10 -15 -20 -20 -25 -30 -35 -25 -40 -30 -45 -50 -35 0 0 0.5 1 1.5 2 Time (s) 12 2.5 3 3.5 0.5 1 1.5 2 Time (s) 2.5 3 3.5 Projectile Motion: DEMO – Throw the ball around Ball has 2-D motion Up & down (called this X above, now call this Y) Direction it is thrown (X) Gravity only acts in Y direction ax = 0 , ay= -9.8 m/s2 = g X = X0 + V0x * t Y= Y0 + V0y * t + ½ * g * t2 DEMO - C2-25: FUNNEL CART DEMO - C2-21: BALLS DROPPED AND SHOT 13 DEMO - C2-22: MONKEY AND HUNTER DEMO - C2-24: WATER DROP PARABOLA End Section 1.1 14 RAMPS: Try pushing something up a ramp. Heavy object sitting on table o Large force Put it on an inclined plane o Less force to move it! DEMO: B2-03: EQUILIBRIUM OF FORCES - INCLINED PLANE You can use a ramp to lift very heavy objects with relatively little force! Mechanical advantage. How does this work? Need to understand addition of forces. What forces are acting me as I stand here? o Gravity down o Why don’t I accelerate? Force of floor acting up Is the force always the same? No, it always just cancels out weight Reaction force 15 Newton’s 3rd law: For every force that one object exerts on a second object, there is an equal but oppositely directed force that the second object exerts on the first object. DEMO: C5-19: ACTION AND REACTION - INSTRUCTOR AND CART Examples Boat on water Person on ice Recoil of a gun We’ll come back to this later in Rockets. Consider a collision between a car and a truck: Mcar = 1 ton Mtruck = 10 ton Ftc = -Fc t Mcac = Mtat ac = -Mt/Mc * at ac = -10 at Summarize Newton’s 3 Laws. 16 Addition of Forces: Draw figure of object with forces from gravity(weight) and the reaction force of the floor. Force is a vector DEMO: B2-02: SUM OF FORCES - SPRING SCALES Give examples of summing of forces. Pushing you east and north, you go northeast DEMO: B2-16: VECTOR ADDITION WITH ROPE AND STUDENTS DEMO: C5-31: AIR TRACK - SAILING UPWIND 17 Work & Energy: The capacity to make things happen is energy, and the process of making them happen is called work. Work & Energy are physical quantities They are measurable! Physical definitions are different then common English usage. Energy o Is not exuberance of a 5yr old o Capacity to do work Work o Not the activities you do to get money o Process of transferring energy Energy is what is transferred, and work does the tyransferring. Important: ENERGY IS A CONSERVED QUANTITY!! We will talk about this a lot all semester. What is work? You do work on an object by exerting a force on it, as it moves in the direction of that force. As you lift a rock you do work on the rock. In both cases you are transferring energy to the object. Sometimes Work (transferring energy) makes an obvious change. Throw the ball o Picks up speed, energy increases Kinetic Energy o Pick up the rock It can do work on the objects beneath it if it drops Potential energy Energy stored in the forces between things Gravitational Potential Energy 18 DEMO: C8-04: HILL TRACK DEMO: C8-11: INTERNAL VS EXTERNAL ENERGY - SPRINGCOUPLED SUPERBALLS DEMO: C8-12: JUMPING MASSES WITH INTERNAL SPRINGS 19 The amount of work you do is determined by how hard you push(force) and the how much distance you push it. Work = Force x Distance How much work do you do in lifting the piano? The work is transferred energy into the piano in the form of Gravitational Potential energy. Gravitational Potential Energy of any object: U m g h Net amount of work you do in lifting the piano is mgh, it doesn’t matter how you get there! If you lift 100kg to a height of 10m U = m g h = 100kg x 9.8 m/s2 x 10m ~ 10,000 kg m/s2 x m = 10,000 Nm = 10,000 Joules (J) Now we can examine how a ramp allows to lift the piano with a little force by giving us a mechanical advantage. Force from ramp Net force weight 20 Small residual net force Ramp supplies most of force needed to keep it from accelerating If you have a 50m ramp go up 5m. for every 10m along ramp you go up 1m. You can push a 2000N weight up this 10 to 1 grade with only 200N of force. The total work is 50m x 200N = 10,000 J It doesn’t matter if you go up the ladder 2000N x 5m = 10,000J or slide it up the ramp with only 200N of force. Either way the final energy (mgh) is the same. Work = LARGE FORCE x small distance = small force x LARGE DISTANCE This ramp gives us a mechanical advantage. SEESAWS: Another form of mechanical advantage using torque. We all now how a seesaw works. Need equal masses. What if we have different weights? Balance it by changing distance from the pivot point. 21 DEMO: B2-32: EQUILIBRIUM OF TORQUES – LARGE DEMO: B3-03: LEVER - WRECKING BAR DEMO: B3-11: PULLEY - HUMAN LIFT 22 Wheels: If we have a heavy object (book uses file cabinet) what happens when we push on it? We start pushing on the file cabinet, but it doesn’t budge. Why Not? Newton’s 2nd Law says if we push it should accelerate Something must be pushing back harder as we push harder to cancel the force. Net force = 0 No acceleration ANSWER: FRICTION – a force that opposes the relative motion of two surfaces in contact with one another. Friction always opposes the relative motion. Microscopic view of friction: Surfaces are not perfectly smooth. Microscopic hills and valleys. o Show figure 1.4.3 from book. Friction depends on Material Force pushing down o F downward force DEMO: C6-03: INCLINED PLANE - FRICTION WITH THREE BLOCKS 23 DEMO: C6-11: SLIDING FRICTION - LECTURE TABLE AND FELT Really two kinds of friction: Static Sliding To start the file cabinets moving you have to exert enough force to overcome static friction. Once it is moving you have to exert enough force to keep it from slowing down and stopping due to sliding friction. Sliding friction is less is generally weaker because once the surfaces are moving they no longer have time to “settle” into one another. DEMO: C6-02: INCLINED PLANE - FRICTION BLOCK WORK, ENEGY, & POWER: Friction wastes energy. Not make it disappear o Energy is conserved Energy can be transferred or converted from one type to another Friction converts useful ordered energy into relatively useless disordered energy 24 Called thermal energy o Energy associated with temperature Called internal energy or heat DEMO I5-02: TRANSFORMATION OF MECHANICAL ENERGY INTO HEAT DEMO: I5-01: MECHANICAL EQUIVALENT OF HEAT - SHOT BAG Remember before we talked about kinetic energy and potential energy Kinetic is associated with the energy of motion More kinetic energy = faster speed Losing energy it slows down o Bowling ball hitting pins 25 o Running back hitting the defense Potential energy is usually not visible Remember gravitational potential energy o Ability to do work by falling down Potential energy is the stored ability to do work 1. 2. 3. 4. 5. Gravitational (ball at top of hill) Elastic (a wound spring or a stretched rubber band) electrostatic (cloud in a thunderstorm) Chemical (a firecracker, gunpowder) Nuclear (Uranium) Energy has units of Joules (J) = Nm = kg m/s2 How quickly you do work or transfer energy is power Power = work/time = joule / s = watt (light bulb!) 1 hp = 745.7 w example: 100kg person up 3 floors(physics building) = 10m U = mgh = 100 x 10 x 10 = 10,000J 1 hp =745.7 w = 745.7 j/s how long to lift person? T = work/power = 10,000J/ 745.7 J/s = 13 seconds 200 hp engine --> .067 seconds -----> 67/1000 26 DEMO: C8-34: POWER - INSTRUCTOR DRAGGING CONCRETE BLOCK Energy goes into friction which dissipates the energy into heat. Wasted energy! Minimize the friction o Wheels!! Move something without sliding using wheels! Once you get it moving there is no friction o Will go on indefinitely with a constant velocity Newton’s Laws! Still some sliding friction Motion between hub and fixed axle Small amount of distance per second o Small radius Means force x dist = work is smaller Further reduce it by lubricationg o Oil Can also use a bearing o See figure 1.4.9 o No sliding friction at all Ball bearings all turn using static friction 27 Linear & Angular Momentum: Measure of an object’s motion or tendency to continue moving in a particular direction. (vectors) Linear momentum (momentum) P=mV Angular Momentum (rotational momentum) L=I Both are further examples of conserved quantities. Kinetic energy KE = ½ m V2 Conserved Quantity Transfer mechanism Energy Linear Momentum Angular Momentum Work Impulse Angular Impulse Conserved Quantities DEMOS DEMO: C2-11: RACING BALLS 28 DEMO: D3-04: ROTATING STOOL AND WEIGHTS DEMO: D3-05: ROTATING CHAIR AND BICYCLE WHEEL DEMO: D4-04: BICYCLE WHEEL GYROSCOPE ON ROPE 29 DEMO: D3-32: KEYWHIP DEMO: D4-22: MONORAIL CAR END WHEELS & THE LAWS OF MOTION 30 Roller Coasters Section 2.3 The experience of acceleration Show DEMO: D1-53: LOOP-THE-LOOP how does this work? A car accelerates forward – what do you feel? you are pressed backward against seat as if gravity was pulling you back as well as down o it is your own inertia resisting the acceleration forward You are experiencing the feeling of acceleration. Roller coasters are the ultimate experience in acceleration. Close your eyes while traveling straight at constant speed can’t feel anything Accelerations no problem feeling it. Accelerating in car feels the same as when you are standing on the floor. The floor supports the weight of your body. The car seat pushes on you to accelerate you forward. When the ground is preventing you from falling you feel your weight as the body senses the forces to support you so you don’t accelerate. When you accelerate in car your body senses the internal forces needed to accelerate you. Which you interpret as weight. The apparent force you experience from acceleration is indistinguishable from the force of gravity. Einstein’s general theory of relativity Draw elevator and digress on Einstein & bending of light The experience during acceleration is called a fictitious force. Points in direction opposite the acceleration 31 Apparent weight is vector sum of gravity and fictitious forces. Figure 2.3.2 from book. Circular motion Merry-go-rounds & spin dryers … Examples of centrifuge A basket or some such thing spinning around a pivot point. Circular motion about a center pivot. What do we know from Newton about motion not in a straight line? There must be a force. o Must be accelerating DEMO: D1-33: ROTATING MASS ON STRING How do we know there is acceleration (force) Can feel the tension on rope What would happen if we let go of the rope? Ball would travel off in straight line o Acceleration is towards the center making it turn and not go off in a straight line. 32 DEMO: D1-31: TRAJECTORY FROM SPIRAL Force keeps pulling in causing an acceleration towards the center. Uniform circular motion Show figure 2.3.3 from book. An acceleration of this type is called centripetal acceleration. Caused by a centrally directed force called centripetal force. The acceleration depends on the speed and radius of the circular motion. v2 a r Now, since the person is accelerating inward towards the center, she experiences a fictitious force outward. This is called “centrifugal force” is a fictitious force If centripetal force is removed the object will go in a straight line Demos of v2/r dependence. 33 DEMO: D1-37: MUDSLINGER Fictitious force is often measured relative to earth’s gravity. 9 = 9.8 m/s2 or 9.8 N/kg In an airplane you may experience a fictitious force >5 times that of gravity or 5 g’s for short. Example: close dryer R = 0.25 m V = 20 m/s A= v2/r = 1,600 m/s2 163 g’s If close had a mass of 5kg really weigh 49N (11 pounds) apparent weight is 1800 pounds!! The water doesn’t accelerate with the clothes which are spun dry. DEMO: D1-52: FAIRGROUND ROTOR 34 Roller Coasters Talk about roller coasters. Lots of accelerations! Discuss various motions and fictitious forces that result. Your apparent weight (g + fictitious force) can be all over! Accelrate up o Very heavy Accelerate down o Apparent force can be less then real weight! Consider accelerating down at just the right rate. Real weight and fictitious upward force just cancel o You will feel perfectly weightless!! What is the rate that causes this? G = 9.8 m/s2 How do get this acceleration? Free fall!! An object not subject to any other forces other then gravity is in free fall (no force on your feet holding you up). Discuss free fall. No weight. Any object with you (sunglasses, pens, etc..)will hover in front of you as you all accelerate together! Similarly, your internal organs don’t have to support each other. DEMO: C4-51: WEIGHTLESSNESS IN FREE FALL - MASS IN BEAKER 35 DEMO: C4-52: WEIGHTLESSNESS IN FREE FALL - MASS IN CUP ON POLE DEMO: C4-53: WEIGHTLESSNESS IN FREE FALL - MASS ON SPRING Now we can talk about roller coasters. Draw figures of loop-the loop and indicate gravity, fictitious force and apparent force. Discuss first versus last car going over the top of a hill like in figure 2.3.9 in book. DEMO: D1-53: LOOP-THE-LOOP 36 ROCKETS (section 5.1) Rockets work on one of the most basic of principles: Action and reaction (Newton’s 3rd law) Propelled forward by pushing material out its tail A rocket gets its thrust by pushing gas out its tail. Thrust is the force that accelerates it forward. Newton’s 3rd law The rocket pushes on the gas so the gas pushes back on rocket. The more gas and the faster the gas is going the more thrust the rocket gets. Recall before when we talked about 3rd Law. DEMO: C5-19: ACTION AND REACTION - INSTRUCTOR AND CART Discuss speed, mass, conservation of momentum Discuss throwing shoes off boat or on ice 37 In a rocket instead of throwing shoes it throws very fast gas molecules. At room temp v=1,800 km/h At 2800 C (5000 F) V = 3 times that High velocity = high momentum o Large thrust! Conventional rocket engine. (figure 5.1.2) Chemical reaction to create VERY HOT exhaust gas. Potential energy stored in chemical bonds of molecules becomes thermal energy. o Thermal energy is mostly kinetic energy in random motion of molecules o Engine’s Nozzle converts random motion into directed motion Permitting gas to escape from only one side The nozzle exerts a net force on the gas. Starts out stationary and ends up going out exhaust The gas pushes back on the nozzle. Overall its own exhaust pushes the rocket forward! It doesn’t need anything outside to push against. DEMO: C5-14: ROCKET TRIKE 38 Will the rocket trike work just as well in the vacuum of space? DEMO: C5-17: ROCKET BOTTLE The space shuttle weighs about 20,000,000 N at launch. The trhust is about 30,000,000 N So, it will accelerate upward. Different kinds of rockets Solid fuel rockets o V ~ 3000 m/s Liquid fuel o V ~ 4500 m/s Model rocket engines at hobby stores. Solid fuel o “C” engine impulse ~ 10 Ns (10 kg m/s (p=mv)) if rocket weighs .1kg V = impulse/mass = 10/.1 = 100 m/s > 200 mph History of Rockets: Date from the 13th century in China. Follow up from gun powder 39 Burning gunpowder sent hot exhaust gas out Used a guide stick to keep it stable. o Like a bottle rocket. Guide sticks eventually replaced by banes attached to sides to give it stability. Liquid fuel rockets suggested later. Solid fuel was reliable & easy to make Liquid fuel offers more chemical potential energy per kg o Liquid fuel can control the thrust 1926 – Robert Goddard launched first liquid fuel rocket. Show figures 5.1.5 & 5.1.7 on solid & liquid fuel rockets. Maximum speed of a rocket:: As long as a rocket can keep pushing material backwards it will accelerate forward and go faster. The speed is limited by how much of its weight is used as fuel. If a rocket is comprised of 90% fuel by weight. The ultimate speed is 2.3 times the gas’ exhaust velocity. To go faster you have to have even higher percentage of fuel. Space bound rockets often accomplish this by using multiple stages. Each stage smaller then the next. Once the 1st stage has used its fuel, the whole stage is discarded and a new lighter rocket begins to operate. Orbiting Earth: Show figure 5.1.8 from book. If something was shot at high enough velocity it would not fall to earth before the earth fell away because of its curvature. 40 At 7.9 km/s (17,800 mph) the cannonball would travel beyond the horizon and would never actually hit the ground. It would circle the earth, returning to the cannon in 84 minutes. Spacecraft can do the same thing if it is going fast enough. The spacecraft will Orbit the earth. Orbit: is a path an object takes as it falls freely around a celectial object. Falling freely: Which way is it accelerating? Center Why? Gravity Near surface g =9.8m/s2 Law of gravitation F G m1 m2 r2 As the spacecraft moves further from earth the gravity is less and the balancing force m2 v 2 m2 ( 2r / t ) 2 m2 ac r r 4 2 41 m2 r t2 G m1m2 2 m2r 4π r2 t2 2 4π t2 r3 Gm1 The larger the radius of the spacecraft’s orbit the longer the orbital period. Space shuttle & most reconnaissance satellites orbit ~200km above earth to avoid the atmosphere. The period here is about 90 minutes. At 35,900 km (22,300 miles) the period is 24 hours. The satellite orbits at the same period as the earth’s rotation Stays directly above a single spot Called geosynchronous orbit DEMO: E1-11: POTENTIAL WELL –MODEL Show pictures of earth from spy satellites. Discuss satellite TV etc…. 42 Heat --- Chapter 6 What comes to mind when I talk about Heat? Hot, cold Temperature We talked about thermal energy before Internal energy of an object o Kinetic energy of atoms & molecules The more thermal energy (kinetic energy of atoms) the larger the temperature DEMO: I6-34: MOLECULAR MOTION DEMO - TEMPERATURE OF A GAS When you touch something hot what you feel is the thermal energy flowing into your hand making it hotter The flow (or moving) of thermal energy is heat DEMO: C8-04: HILL TRACK Discuss motion of ball around potential minimum Kinetic(thermal) energy of molecules The more energy (temp) the larger the amplitude The temperature can effect the properties o Size, pressure etc…. 43 DEMO: I1-12: THERMAL EXPANSION - BALL AND RING DEMO: I1-13: THERMAL EXPANSION - BIMETAL STRIP DEMO: I4-17: AIR BALLOON ON LIQUID NITROGEN DEMO: I1-52: TUNING FORK AT LIQUID NITROGEN TEMPERATURE 44 Temperature tells you which way heat will flow between two objects. From hot to cold DEMO: I1-42: THERMOELECTRIC FAN Until thermal equilibrium is reached o No heat flow when in thermal equilibrium DEMO: I2-27: THERMAL EQUILIBRIUM BETWEEN ALUMINUM AND COPPER Temperature scales: Celsius Fahrenheit Kelvin 45 Freezing 0 32 273 Boiling 100 212 373 Abs zero -273 -460 0 Wood Stoves How do we produce heat? Fuel: Chemical reactions to release energy? DEMO: I1-63: HYDROGEN EXPLOSION Burn wood Hydrocarbon molecules o H,C,O o Carbohydrate Hydrocarbons + O2 H20 +CO2 o Reaction products Have lower potential energy then the original molecule Activation Energy: Energy needed to break molecular bond. Then the release of energy by going to the lower energy reaction products is released. Also used to keep the reaction going for other molecules. Light the wood then it keeps burning Use Hill track demo again activation energy released energy 46 Better Fuels are : Kerosene Natural gas o Pure hydrocarbons Hydrogen o 2H2 + O2 2 H2O How does the Wood stove heat the room? How does heat move? Remember the DEMO on thermal equilibrium? Conduction o Contact o Heat fows from hot to cold How does it flow? Energetic molecules and atoms hit their neighbor o So-on and so-on Bucke-Brigade Electrons are free to move in metals (mobile) o Better conductors of heat And electricity Generally, good conductors of electricity are good conductors of heat. Copper, silver, gold, aluminum etc… Bad conductors Air, plastic, wood, glass Exception Diamond o Good substrate for electronics Back to the wood stove: Heat flows from inside the stove to outside the stove walls by conduction. metal o Outside surface gets very hot! What carries heat to room? o Air is a very poor conductor!!! 47 Convection & Radiation: Convection: Heat moving with air Warm air rises o Causes a flow of air in room o Show figure 6.1.5 The air heated by contact with the hot stove Temperature lowers density of hot air Floats upward, pulling cold air behind it to replace it Eventually heated air cools and descends down to floor Drawn back to hot stove to repeat cycle Creates a Convection Current. Looping path is called a convection cell. Sometimes needs help: Adding a ceiling fan will bring the warm into the entire room faster. DEMO: I2-43: CONVECTION - HOT PLATE 48 Radiation: How do you get warmed by the sun? How does heat get here from the sun? No conduction No convection Radiation! Hot objects radiate energy! Electromagnetic waves o Light, radio, tv, x-ray, microwave etc… o Carry energy, travel through empty space at the speed of light o Can carry thermal energy Thermal energy will flow (heat) from a hotter object to a colder object via radiation. Needs no medium between objects Show electromagnetic spectrum (figure 6.3.2) DEMO: I2-01: CROOKES' RADIOMETER The type and amount of radiation depends on the temperature of the object. Thermal Radiation 49 Colder object may only emit radio or infrared Hotter object can emit visible light The red glow of the hot coals in the stove! Hotter objects emit more thermal radiation Eyes are only sensitive to visible light. We can’t see all the thermal radiation Show blow up of em spectrum in visible (figure 6.3.3) DEMO: I2-06: THERMOPILE WITH AUDIO OSCILLATOR DEMO: N1-05: SPECTRA - VISIBLE AND INVISIBLE 50 Eyes see visible light from sun (5800 C) Big Bang --> Microwaves (<3 K) Body temperature --> Infrared Imaging for detecting people and night vision Heat (Thermal radiation is like light) Can be focused Mirror DEMO: L3-16: FOCUSING OF HEAT WAVES BY MIRRORS Clothing & Insulation: Why do we lose heat? Body temperature is higher then surrounding How do we maintain temperature instead of reaching equilibrium? o Warm blooded o Produce heat to maintain higher temp T= 37C (98.6F) T means heat flow 80 cal/hr = 100 watts (light bulb) Reduce or minimize heat loss Conduction o QkA T X Thick 51 Bad conductor Air, fat, skin Smaller T Lower temp in hands and feet o Feels cold Convection o Heat warms air around you o How quickly? Specific heat capacity Air is 4 times water Why you cool off (lose heat) faster swimming o Air is a poor conductor (remember wood stove) Skin warms only a small layer If air remains still wouldn’t lose much heat o Convection removes the warm air keeps T higher o The enhanced heat loss by moving air Wind chill o Keep air from moving Insulation with air pockets Feathers Dual glass panes DEMO: I2-09: DEWAR - TRANSPARENT WITH LN Radiation o Amount depends on T and how well they absorb/emit L T4 52 Tsun = 6000K Tsun/Tme = 20 Tme = 300K 204 ~ 160,000 times more heat towards me Night Sky --> T ~ 0 Lose about all of the 100w to the dark sky Reduce heat loss by standing under a tree Black is a good absorber of radiation Mirror is a good reflector (remember match demo) Insulator to thermal radiation heat transfer DEMO: I2-10: DEWARS - SILVERED AND UNSILVERED Keeping cool – sweat Evaporative cooling Buildings: Air insulation Avoid convection 53 Trap air in porous fibers o Glass wool, foam, etc… Ceiling is important because the hot air rises Windows o Air space o Double pane windows Gas- air or argon Law of thermal radiation: “Black Body” Radiation Black Body Perfect radiator Perfect aborber Need quantum mechanics to understand (led to quantum) Light comes in indivisible packets (quanta) of energy E = h per “photon” – quantum of light Intensity of a given color given by number of photons 54 8h 3 E ( ) 3 h / kT c e 1 Higher Temperature Higher overall intensity Color is more blue then red DEMO: I2-04: WIEN'S LAW OF THERMAL RADIATION END Lecture 55 Incandescent Light Bulbs Light is electromagnetic radiation Thermal radiation Hot wire filament in a light bulb emits visible light as part of its thermal radiation (show light bulb figure from 6.3) DEMO: L1-02: TUNGSTEN-HALOGEN LAMP - PROJECTION OF LAMP Remember discussion of thermal radiation. Most is invisible to our eyes Distribution of wavelengths Eyes sensitive to a narrow window of wavelengths o Show slide with figures 6.3.2, 6.3.3 Any object with T>400 C emits enough visible light to be seen in a dark room At higher temperatures it brightens and color shifts from red to orange to yellow to white To reproduce sunlight the filament would have to be 5800C. Tungsten Filament Tmax = 2500 C o Can’t really reproduce sunlight color Remember black body spectrum 56 Show figure 6.3.4 of wavelength distributions for 3 temps Show table 6.3.1 discuss color temperature Object Heat Lamp Candle Flame Bulb Filament Sun’s Surface Blue Star Temperature 500 C 1700 C 2500 C 5800 C >6000 C Color Dull Red Dim Orange Bright yellow-White Brilliant White Dazzling Blue-White Notice from figure 6.3.4 there are 2 principal shortcomings of Incandescent light bulbs Poor efficiency at converting electrical energy (heat) into visible light Low temperature color The Filament: The filament is made hot by giving it thermal energy (heat). The energy comes by passing electrical current through it. Current consists of electrically charged particles Most of their energy is converted into electrical energy o Kind of like thermal energy getting created by dissipating the kinetic energy by friction resistance DEMO: K5-32: RESISTANCE VS DIAMETER AND LENGTH 57 Filaments First filaments were Carbon o 1879 Thomas Edison bulb survived for several hundred hours o carbon has highest melting temp (3550 C) o However, it evaporates directly from solid to gas very quickly Sublimation DEMO: I4-51: SUBLIMATION OF DRY ICE – PROJECTION o When enough sublimates away a gap occurs in filament. It stops carrying current Bulb “burns out” Better choice id Tungsten o Melts at 3410 C o Sublimation very low o Can be run at higher temps then carbon before sublimation rate is too high o Hotter = brighter, whiter light How do you make a long and thin filament to get enough electrical resistance? A typical 60 Watt light bulb 2 meters of 25 micron (.001 in) tungsten wire o wound into a double spiral first wound into a thin spring-like coil then the coil is wound into a coil itself 58 see Figure 6.3.5 not accomplished until 1937 To keep the white hot filament from burning or subliming quickly the surrounding gas cannot be air. If air (oxygen) is present it will burn immediately DEMO: L1-03: LIGHT BULB WITHOUT VACUUM Need to remove air or fill with inert gas Mostly argon No Oxygen Gas wastes energy by allowing heat to escape from filament by conduction and convection. Dark spot on top of bulb from convection currents carrying tiny tungsten particles Krypton is better then Argon 59 Poor heat conductor Persistence of light Current is 60 cycle per second AC o Current goes on and off 60 times a second Why does the light appear continuous? o Filament doesn’t cool off that fast o 60 cycle is faster then eye can see DEMO: L1-05: PERSISTENCE OF A FILAMENT Extended Life and Halogen Bulbs Extended life longer filament o doesn’t get as hot won’t sublimate and burn out as quickly lower temps means less fraction of visible light o can make it so long that it doesn’t get hot enough to give off any visible light Extended life bulbs are less energy efficient! Halogen Bulbs Run the filament hotter 60 brighter light closer to sun’s color temp more energy efficient sublimation much higher o burns out quickly Photoflood lamps for photography Halogen bulb uses a trick to “rebuild” the filament as it sublimates to allow it to last longer with the higher temps. Encased in a quartz tube o Tolerate the higher temps and chemicals Contains molecules of a halogen (bromine, sometimes iodine) Halogen reacts with tungsten to make stable tung-halogen molecules that don’t stick to glass These molecules drift around tube until they contact hot filament Then torn apart and the tungsten sticks to the filament thus rebuilding the filament The rebuilding process is not completely uniform. Eventually the filament will get a break and burn out, but after a much longer time Halogen bulbs run at much hotter temperatures. Brightness of bulb related to amount of heat energy per second to heat up filament. Heat energy comes from electrical energy Energy/s = J/s = watts o 60 W, 100W bulbs … We have talked about incandescent bulbs bulbs that emit light by thermal radiation 61 There are other kinds of lights fluorescent laser they use quantum mechanics o transitions between energy levels in atoms o electron goes from a higher potential to a lower potential o conservation of energy! Energy is converted to light energy (photon) fall back down emit photon (light) redder raise energy bluer DEMO: L1-32: VISIBLE LASER DEMO: N2-02: DIFFRACTION SPECTRA - THREE SOURCES EXPENDABLE GRATINGS 62 Thermodynamics -- Chapter 7 heat flows from hot to cold rules governing the movement of heat Study by examining 2 everyday machines 1. Air-conditioner 2. automobile engine Talk about different kinds of energy. Not all are the same ordered disordered AC ordered electric energy to transfer heat from cold to hot (against its normal flow) Auto thermal energy can be used to do work (ordered energy) but at a cost 7.1 Air Conditioners want to remove thermal energy from something (e.g. room) transfers heat against its normal direction of flow requires ordered energy (electricity). Why? How can we cool off our house on a hot day? 1. Let heat flow from yours to someone else’s house? o 0th Law – 2 objects(houses) in thermal equil. With a third(air) are in thermal equil with each other. o Won’t work. 2. Destroy the thermal energy in the house 63 o Conservation of energy won’t allow you to do this. o 1st Law – o u = Q + W explain in words Show some adiabatic Q=0 processes to illustrate U = W DEMO: I5-11: ADIABATIC PROCESS - AIR PISTON WITH THERMISTOR DEMO: I5-13: ADIABATIC EXPANSION OF AIR - GRAPH OF TEMP DEMO: I5-15: ADIABATIC EXPANSION OF CARBON DIOXIDE 64 Still doesn’t explain some things heat flowing from cold to hot o pond freezing on a warm day weight cooling off and gaining kinetic or potential energy also, number 3 in ways to cool off house 3. Convert thermal energy (heat) to electric energy and cool off house Why won’t this work? o Burning log example Won’t re-assemble very improbable (0) o Once energy is scattered randomly among individual particles (thermal energy) it won’t collect back again spontaneously o Ordered and thermal energy are not the same o There is some other law we need to describe this New Law of Thermodynamics (2nd Law): Disorder of an isolated system never decreases. System that starts out ordered ends up more disordered Name: total disorder = Entropy Heat carries entropy. The more heat the more disorder because of the higher amount of random energy (thermal energy). A weight cannot lower its temperature and raise itself in gravity Doesn’t violate energy conservation But, the disorder (entropy) would go down. o Lower temp = lower heat = lower disorder DEMO: I5-41: ENDOTHERMIC REACTION – ENTROPY 65 This law of entropy is an empirical law. Fact of nature Like the arrow of time No known physical reason why it is this way! o Microscopic laws are all reversible o Connected to time, quantum collapse?? Gravity?? This Law explains why you can’t make a perpetual motion machine. AC lowers the entropy of the house (cools it off) we must somehow raise the entropy somewhere else even more then we lowered it in the house. Let see how this works mathematically S = Q/T Entropy in normal heat flow from Hot to cold S = Q/T HOT COLD TH TC SH = -Q/TH SC = Q/TC STOT = Q(1/TC – 1/TH) > 0 The heat entering the colder system creates more disorder in it then order in the hotter system. Example of tea party two rooms with bunch of kids (lots of energy = high temp = more disorder) and one with a tea party of adults (low energy =low entropy). Let door open, flow of 1 kid into tea party. Creates more disorder in the tea party. The room with the kids doesn’t get much more ordered! 66 AC does the seemingly impossible. How? COLD Heat Pump Hot ^ | ordered energy in converted to heat(disordered) to increase entropy. How do we put in ordered energy? Work or Power o Electricity, fuel … COLD Heat Pump Hot ^ | WORK S = Q/T HOT COLD TH TC SH = +(Q+W)/TH SC = -Q/TC STOT = Q(1/TH – 1/Tc) + W/TH > 0 <0 need work (ordered energy = electricity) to make entropy increase! Nothing is for Free!! How do we make this happen in an actual AC? Working Fluid Transfer of heat Absorbs heat from inside Absorbs ordered energy (work) (pump) Releases heat and from inside heat taken + work converted to additional disordered energy 67 3 main components Evaporator - inside – takes heat Condenser - outside – outputs heat Compressor - usually outdoors – does work on fluid Show figure 7.1.2 from book and describe cycle High pressure liquid Constriction High pressure in fast low pressure out Low pressure it evaporates and cools E.g. sweating evaporates DEMO: I5-15: ADIABATIC EXPANSION OF CARBON DIOXIDE Cold liquid cold gas in evaporator Temp drops Warm air in house is blown over evaporator coils by fan Takes heat away by conduction air cools Gas goes from cold to cool Cool gas leaves coil Compressor Takes cool high pressure gas in Compresses it - does work Adds heat out comes high pressure hot gas 68 DEMO: I5-11: ADIABATIC PROCESS - AIR PISTON WITH THERMISTOR Draw diagram of how pump works on board. DEMO: F4-52: FORCE PUMP – MODEL Does work on gas W = P dV U = W = P dV Temperature goes up! Hot gas goes through condenser outside o Cools off because it is now hotter then outside air o Hot gas goes to warm high pressure liquid Start all over again in cycle 69 Pumps heat out of house. Adds heat from work done. Pumps heat + heat from work to outside. Overall entropy goes up. You must add ordered energy, or work, to make heat flow from cold to hot! The Fluid itself. Gas at low pressure Liquid at high pressure Chloro fluorocarbons (Freons) o Replaced ammonia Toxic Corrosive Ozone layer is eaten by chlorine. Hydro fluorocarbons replaced by non-chlorine molecules Not as energy efficient! 7.2 Automobiles Engine: Thermal Energy Ordered Energy (work) Avoids conflict with the entropy Law by being a heat engine Get work out of disordered thermal energy as heat flows from hot to cold. Some heat is wasted in flow of hot to cold thus increasing entropy overall. Can’t make a 100% efficient engine. HOT COLD | |- Work = QH - Qc such that overll entropy increases. some heat must flow into COLD. Internal Combustion Engine , see figure in book & gif on WEB. 70