Multimedia presentation - ICAR-CNR
advertisement

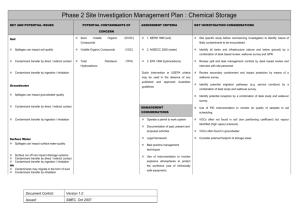
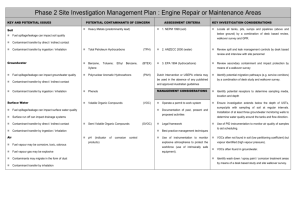
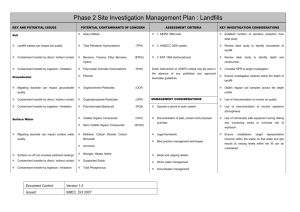
In-situ bioremediation is based on the use of bacteria to degrade the contaminant directly in the polluted soils, and can be seen as a way to improve and accelerate natural decontamination processes. As it is usually based upon the use of indigenous bacterial strains, it is environmentally safe and less expensive than other decontamination techniques. Generally speaking, there are some problems in order to predict the outcome of field scale operations from laboratory or pilot plant data and to reliably evaluate the times and the costs of the intervention; the COLOMBO model is developed to describe the time evolution of the relevant variables during bioremediation and to solve this kind of topics. We often have to deal with contaminants not miscible with water or only slightly soluble, as mineral fuels (e.g., petrol, fuel oil) or solvents. For this reason, the COLOMBO model can simulate a whole bioremediation process in a three-phase water-oil-air system. The model is based on a straightforward discrete scheme, namely that of cellular automata (CA), which rely upon discrete space cells, discrete time steps and discrete state space. In this approach, the interactions are local, the state of each cell at time t+1 - x(t+1) depends upon its own state and those of its neighbours at time t and a transition function leads from the old cell_state to the new cell_state. The model has a layered structure: In the fluid dynamical layer, we deal with the phase movements inside the soil. The basic idea is that the pressure waves (and the relative potential distribution) reach the steady state “suddenly” with respect the phase flows, and so they can be computed before everything else; then the flows of each phase in the porous media is calculated following the gradient of potential. In order to follow a real experiment or field intervention, the model is able to simulate saturated or unsaturated zones, external forced condition like wells with fixed infiltration (exfiltration) rate and physical boundaries like surfaces or impermeable walls. An example of fluid dynamical layer simulation, in which one injection well (left side) and one extraction well (right side) are present into a saturated soil; water is introduced from the injection well to a constant pumping rate. Once the pressure field has reached a steady state, the model computes the water potential distribution (on the left) and then the water flow (on the right). Once the flows of each phase have been determined, the chemical layer can simulate the fate of the contaminants. The model computes the changes in concentration over time due to advection, (in which dissolved chemicals are moving with the flowing phase where they are present), hydrodynamic dispersion (in which molecular diffusion and small scale variations in the velocity of flow through the porous media cause spreading of the contaminant front), exchange among phases (e.g. adsorption/desorption phenomena) and chemical reactions. In the last case, we simulate the dissociation of hydrogen peroxide in oxygen and water. The exchange among different phases can be modeled both assuming an instantaneous equilibrium and dynamically modeling a couple of matter flows through each interface between different phases, to reach a steady state. An example of chemical layer simulation. The fluid dynamical configuration is the same of the previous example; two contaminant zones are present in the soil matrix. We can observe two kind of phenomena: first, the soluble components of the contaminant pass from the soil matrix to the water and second, they are transported from the water flows towards the extraction well. The contaminant concentration in the soil decreases as time passes. In the biological layer the model simulates the growth of the biomass and its interaction with nutrients and contaminants. We suppose that, potentially, the bacteria could utilize the contaminant presents in each phase of the ground; the electrons acceptor is the oxygen dissolved in the water. The model can simulate a biodegradation process by both aerobic and anaerobic reactions. In the first case, it uses an oxygen-limited reaction following a Monod kinetic to describe the growth of the biomass. In addition to oxygen, other nutrients, such as nitrogen and phosphorous, may limit the biodegradation of the contaminants: the model takes account of this fact. In the second case it is assumed a time constant decay of the interested chemical. In the figures we can see a case study of application of the whole model, in which we deal with a soil portion of a real intervention. The field is been divided in hexagonal zones with a side of 20 meters; on the vertexes of each hexagon and on its center there are extraction wells while inside the hexagons there are 18 injection wells. We have simulated one hexagon: water enriched with hydrogen peroxide is introduced by means of the injection wells. The oxygen, produced from the hydrogen peroxide dissociation, is carried by the water flows (which follow gradient of the water potential distribution). It should be noted that, where the oxygen presence is bigger, we have the highest bacteria growth and the smallest contaminant concentration. Fluid dynamical layer The multiphase flow in a porous medium is governed by Darcy law: q k r k p gz (1) where q (m/s) is the volumetric flow, k (m2) is the permeability of the solid matrix, kr is the relative permeability of the phase , ma (Pa s), p (Pa) and (kg/m3) are the viscosity, pressure and density of phase respectively. kr takes into account the presence of different phases in the soil and depends of the saturation S (defined as the ratio between the volume of the phase and the pores volume in a reference volume). The phases present into the soil can have different affinity with the soil matrix: they could tend to stay near the surface of the soil particles (wetting phase), or they could tend to stay far from the surface of the soil particles, in the middle of the pores of the soil (non wetting phase). In a two-phase system there is a correlation between the pressures of the wetting (pw) and the non-wetting phases (pnw), expressed in terms of the capillary pressure pcnw (the pressure discontinuity at the interfaces between the wetting (w) and the non-wetting (n) phases): pn = pw+ pcnw(Sw) (2) The capillary pressure can be itself be expressed in terms of the saturation of the wetting phase Sw, following the Van Genuchten model. In a three-phase gas-NAPL-water system we can write: pn = pw+ pcnw(Sw) pg pn = pcgn(Sg) = pcgn(Sw , Sn) (3a) (3b) where pn (Sn), pw (Sw) and pg (Sg) are the saturation pressures of the non-wetting phase (NAPL, non aqueous phase liquid), water (the wetting phase, in this case) and gas respectively. The relative permeability too depends on the presence of the different phases. In a two-phase system the relative permeability of each phase can be expressed in terms of the effective saturation Se of the wetting phase, following the approach of Mualem and Van Genuchten (MVG). In a three-phase water-oil-gas system, the preliminary experiments for the determination of the relative permeability show that the relative permeability of water depends only on the saturation of the water itself: due to its highest affinity to the solid matrix, the water fills the smallest pore space, no matter how the remaining pore space is subdivided between oil and gas. The relative permeability of oil and gas in a three-phase system, however, depends on the saturation of all three phases. The reason is that, in a two-phase system with water, oil fills the larger pores, whereas, in a two-phase system with gas, it fills the smaller pores. Based on these considerations, different authors have developed methods for determining the relative permeability-saturation without measuring the three-phase system. In the COLOMBO model, we have followed the model of Stone II. The transition function The basic idea is to consider as reference pressure the water pressure: the pressures of the other phases are obtained by applying relations (3a) and (3b). Pressure waves move faster than phase flows so we can suppose that phases moves only after that the pressure field has reached a stationary state (that depends on the phases distribution in the soil). Therefore, the following procedures define the global evolution of the system: a) the update of state variable values imposed from outside (e.g. the flow rate of the pumps); b) the computation of the phase potentials (it involves the application of the transition function for the pressure several times to CA); c) the computation of the flows; d) the computation of the remaining state variables. Particularly, mass conservation requires that the algebraic sum of the flows in a cell vanish: f 6 k 1 i k f ko 0 (4) where f ki means the incoming flux of any kind of phase, and f ko means the outcoming flux of any kind of phase; the possible flux direction are six (k ranges from 1 to 6) because of the topology of the neighbourhood. By inserting equation (1) and (3a) in (4) (and assuming, for simplicity, that only air and water are present inside the soil), we can derive: k 0rw k p wj w gz j k 0ra k p wj p cwa a gz j j cell _ size a cell _ size j 1 w 6 0 (5) with: p wj p wj p0w z j z j z 0 (6) where the subscripts w and a means, respectively, water and air; p is the pressure, the density, the viscosity. This equation depends only upon the values of the water pressure; we can derive the value of the water pressure of the central cell as a function of the pressures of water in the neighbouring cells. This is exactly the definition of transition function: the value of a state of a cell depends upon the values of the neighbouring cells. p w0 f p w1 , p w 2 , p w3 , p w 4 , p w5 , p w6 (7) This transition function is iterated over the whole cellular automata until a stationary condition is reached. From the potential distribution we can compute the pressure distribution by subtracting the gravitational potential and the phase flows which follow the gradient of the potential. Return to the COLOMBO model Chemical layer The most relevant phenomena that can involve chemicals are: advection, due to the motion of the phase in which it is present; hydrodynamic dispersion and molecular diffusion within the phase that contains the chemical; exchange among phases (e.g. adsorption/desorption, transpiration); chemical reactions. Return to the COLOMBO model Biological layer The biodegradation of an organic compound is the result of many different processes that can be affected by many different factors: the presence of other microorganism's (fungi, actynomicetes etc), different kinds of bacteria (that can cooperate or compete), chemical compounds, chemical and physical characteristics of soil, temperature etc. etc. The main factors that describe the behavior of a bacteria population are: Spontaneous growth and death; Decrease due to the presence of poisonous chemicals; Growth due to the degradation of a specific compound. COLOMBO model describes aerobic biodegradation in a contaminated soil. The proposed model can describes the behavior of three kinds of bacteria: degrading bacteria (which are able to degrade the contaminant), resistant bacteria (which can survive in presence of the contaminant) and non resistant bacteria, for which the contaminant is poisonous. Of course, the behaviour of the degrading bacteria is the most relevant in bioremediation. We suppose that, potentially, the bacteria could utilize the contaminant presents in each phase of the ground; the electrons acceptor is the oxygen dissolved in the water. Bacteria Contaminant exchange Oxygen exchange We can show the biological transition functions that describes the evolution of a bacterial population X and the relative consumption of contaminant. If the degradation phenomenon is not aerobic, we can model the decrease of the pollutant concentration by means of a time constant decay. Return to the COLOMBO model