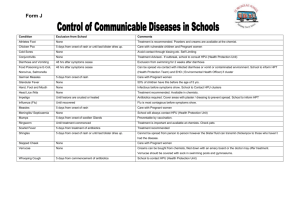
Joint Infectious Disease Protocol 2012
advertisement

Health Protection Agency North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL London Boroughs of Barking & Dagenham, Barnet, Camden, Enfield, Hackney, Haringey, Havering, Islington, Redbridge, Newham, Tower Hamlets, Waltham Forest and the City of London Current version V2.2 This document was reviewed by Maria Saavedra-Campos, Katie Fleet, Sarah Addiman, Erika Huszar and the EHOs of the EHDs above North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 OCUMENT NAME FILE LOCATION FOR USE BY JOINT INFECTIOUS DISEASE PROTOCOL DOCUMENT STATUS CURRENT VERSION ISSUE NUMBER AND DATE Final plan document V2.2 September 2012 V2. September 2011 V2.1 January 2012 April 2006: V1. Author: Sarah Addiman AUTHORS Health Protection Agency. London NENC Local Authorities (Environmental Health Officer) September 2011: V2.1 Revision – Maria Saavedra-Campos, Katie Fleet, Sarah Addiman, Erika Huszar September 2012: V2.2 Reviewed by Maria Saavedra-Campos - 9.3 section added management of sporadic cases of campylobacter and salmonella spp. & updated actions table - Updated version of London Outbreak Plan - Shigella questionnaire OTHER CONTRIBUTORS CONSULTATION (September 2011) North East North Central London HPU EHOs Local Authorities in North East North Central Sector ACCREDITATION AND ENDORSEMENT NENC London HPU Environmental Health Departments North East & North Central London Sector REVIEW DATE September 2013 Page 2 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Contents 1. INTRODUCTION ......................................................................................... 4 2. AIMS OF THE INVESTIGATION OF INFECTIOUS DISEASES OR CONTAMINATION ........................................................................................... 5 3. SURVEILLANCE.......................................................................................... 5 4. COMMUNICATION OF SPORADIC CASES OF INFECTIOUS DISEASES 5 5. COMMUNICATION OF LINKED CASES, CLUSTERS OR SMALL OUTBREAKS ................................................................................................... 6 6. MAJOR OUTBREAKS ................................................................................. 7 7. COMMUNICATION OF CROSS BOUNDARY CASES OF INFECTIOUS DISEASES ....................................................................................................... 8 8. COMMUNICATION OF SITUATIONS WHERE CONTAMINATION IS IDENTIFIED ..................................................................................................... 8 9. MANAGEMENT OF ROUTINE SPORADIC CASES ................................. 14 9.1 INTRODUCTION ............................................................................................. 14 9.2 RISK ASSESSMENT ........................................................................................ 14 9.3 MANAGEMENT OF SPORADIC CASES OF CAMPYLOBACTER AND SALMONELLA SPP NON TYPHOID OR PARATYPHOID ............................... 15 9.4 MINIMUM AGREED ACTIONS ..................................................................... 16 9.5 FURTHER INVESTIGATION ......................................................................... 17 9.6 SPECIMEN COLLECTION .............................................................................. 17 9.7 CONTROL......................................................................................................... 18 9.7.1 General advice ............................................................................................ 18 9.7.2 General advice in relation to gastrointestinal disease ................................. 19 10. DEALING WITH SINGLE URGENT CASES ............................................ 19 11. REFERENCES ........................................................................................ 21 Appendix 1a – Disease Information ............................................................... 22 Appendix 1b – Toxins .................................................................................... 60 Appendix 1c – Factsheets, links and policies ................................................. 71 Page 3 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 JOINT INFECTIOUS DISEASE PROTOCOL 1. INTRODUCTION Significant changes to Legislation as outlined by the Health Protection Legislation (England) Guidance 2010 (Appendix 1c) have encouraged the revision of the 2006 Joint infectious disease protocol. The document is intended for use as an initial guide, to supplement management of individual cases of infection and complement existing “inhouse” procedures. Thus fulfilling the agreement laid out in the joint Memorandum of Understanding. In some cases it will be necessary to refer to supplementary detailed guidance. Where this is the case this has been indicated in the text. This document summarises the working arrangements between the North East and North Central London Health Protection Unit (NENCL-HPU) and the Environmental Health Departments (EHD) of the Local Authorities (LA) in the sector regarding the investigation and communication of infectious diseases. The document includes relevant organisms that might not necessarily be notifiable according to the Health Protection Legislation (England) Guidance 2010 but that might pose a public health risk. The guidance explains the requirements on registered medical practitioners to notify cases of infectious disease or contamination (chemicals or radiation) that present a significant risk to human health and the requirements on diagnostic laboratories to notify the Health Protection Agency (HPA) of specified causative agents. Note that the focus of the document is on infectious diseases and notifications of organisms. However, communication arrangements regarding contamination notifications have been included as this has now been included in the new legislation. The document illustrates: how the two departments will communicate depending on the urgency of the diseases/organisms or contamination notified roles and responsibilities and clarification on who will be leading the investigations of specific diseases, situation or outbreaks a list of minimum actions required for sporadic cases of food-borne pathogens surveillance outputs that they HPU will provide on a regular basis This is based on previous agreement in the Joint Infectious Disease Protocol (2006) and EHD forums, new health protection legislation (2010) and ongoing organisational changes in both, NENCL-HPU and EHDs. Page 4 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 2. AIMS OF THE INVESTIGATION OF INFECTIOUS DISEASES OR CONTAMINATION To prevent the spread of infection or further exposure to a contaminant and to reduce the number of cases of illness; To ensure the prompt recognition and identification of the source of the infection or contamination; To initiate appropriate action and provide any advice and education which is necessary to control the source of the infection/contamination and to prevent a recurrence 3. SURVEILLANCE The HPU will continue to produce weekly, monthly and annual reports that will be circulated to the EHDs by email. The weekly reports will include cases, incidents and outbreaks in the sector notified to the NENCL HPU and for which the investigation was primarily lead by the HPU. This report is produced from HPZone data and does not currently include laboratory notifications forwarded directly from the HPU to EHDs for action The monthly report includes all cases, incidents and outbreaks notified to the NENCL HPU and entered on HPZone. It also includes some comparisons with previous years. It is planned that monthly reports will also include laboratory data, mainly including infections for which the investigation is primarily lead by the EHDs i.e. campylobacter An annual report will also be produced and distributed to the EHDs in the sector. This will include data from both data sources HPZone and lab data. None of these reports will contain personal identifiable information (PII). The reports provide an overview of the activity in the sector. Enhanced surveillance will continue to be routinely required for certain diseases (e.g. Listeria). This may take the form of a national enhanced surveillance questionnaire e.g. Legionella, enteric fever or it may be specific to certain infectious diseases in the event of an increased in the national incidence e.g. Salmonella spp. There may also be occasions where the EHD and HPU have agreed to carry out local enhanced surveillance for a particular communicable disease. 4. COMMUNICATION OF SPORADIC CASES OF INFECTIOUS DISEASES The document contains two tables with a list of relevant diseases, organisms and syndromes or clinical presentations. Note that table 1 and 2 refer to Page 5 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 sporadic cases. Table 1 is organised alphabetically by disease. The tables contain how quickly the information should be passed between departments as agreed between EHDs and NECL HPU in July 2010. The tables also provide information on who leads the investigation i.e. HPU, EHD or joint. For the purpose of communication it was agreed that urgent cases should be communicated on the same day. Urgency would be determined by the following: o diseases for which immediate action is needed to ensure that appropriate public health actions are instigated e.g. enteric fever o diseases that fall within the remit of joint working between EHDs and HPU team before investigations and control are agreed (Table 1 and 2) o diseases that might attract significant media attention e.g. anthrax, Viral haemorrhagic fever o diseases for which the EHDs lead the investigation and early intervention is necessary to prevent further exposure e.g. food poisoning link to a particular establishment Some diseases and organisms are associated with major concerns, even if they are sporadic cases, in this instance communication between departments should happen immediately. For urgent notifications communication would be on the same day the notification is received. As specialist assessment is required to be undertaken by the EHD and the HPU it is essential that notification of urgent cases is communicated early. NENCL HPU will forward lab reports of notifiable infections requiring routine EHD action via encrypted email on a “next working day” basis (via fax for EHD Hackney until the encrypted system is set up). Note that urgent cases will be called through for discussion and to agree further actions. Documentation related to urgent cases i.e. questionnaires, potting schedules would be sent via encrypted email (via fax for EHD Hackney until encrypted system is set up) unless advise otherwise. Note that email addresses will be kept in the contact lists folder. It is the responsibility of both organisations to keep each other informed of any changes to email addresses or contact numbers so prompt communication and continuity of service can be maintained. Note that for conditions not covered or where there is any uncertainty both organisations should consult each other as a matter of urgency to determine whether further action is required and to agree roles and responsibilities. 5. COMMUNICATION OF LINKED CASES, CLUSTERS OR SMALL OUTBREAKS For diseases not circulating in the community, an outbreak is two or more persons with the same disease or the same organism isolated from a Page 6 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 diagnostic sample, or who are linked through common exposure, personal characteristics, time or location. For diseases circulating in the community, an outbreak is a greater than expected rate of infection compared with the usual background rate for that particular place or time. The commonest diseases, which give rise to an outbreak, are gastro-intestinal infections (bacterial or viral). Sometimes a single case of certain diseases such as Diphtheria, rabies or Viral hemorrhagic fever, where there is an immediate or serious health hazard to the population of a Health/Local Authority (LA) may be managed as an outbreak. When organisations become aware of a potential outbreak they should immediately communicate with each other. Environmental Health Department initial action EHDs becoming aware of outbreaks should ring NENCL - HPU and speak directly with the on-call team. EHDs will then implement actions specified in their own in-house procedures and work instructions. HPU team initial action If the HPU team are informed first (for example by GP or microbiologist) they should ring the relevant EHD immediately. Allocate a person or team to respond Circumstances will dictate how an episode will be recognised by the HPU and there may be difficulty in deciding when to declare that a significant outbreak has occurred. Suspicions may be aroused as a result of increases in notifications of infectious disease and/or laboratory reports as well as on information received from Hospital Consultants, GPs, EHOs, Water Company representatives, members of the public, etc. Revision of surveillance data and trends will assist on making this decision. The HPU will seek advice from the Regional Epidemiologist or from the Microbiology services as appropriate. The principles and procedures of the outbreak response are included in the London Infectious Disease Outbreak Management Plan (Appendix 1c). 6. MAJOR OUTBREAKS A more serious outbreak may be an outbreak where there are a large number of cases or one where the illness is severe, such as to require hospitalisation. Major outbreaks may require EHDs and/or HPU Incident and Emergency plan to be activated. The incident will be managed by an outbreak control team with similar composition as for smaller outbreaks. However, representatives from other geographical areas and specialist advisers are likely to be required. Page 7 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Sufficient office accommodation and administrative support is needed. This may need to be within a major incident room (either at a LA, NENCL-HPU office or a local Public Health Department site). 7. COMMUNICATION OF CROSS BOUNDARY CASES OF INFECTIOUS DISEASES This refers to cases for which initial investigations show that possible sources of infection or exposures are identified in different LA e.g. food poisoning cases link to several food establishments. In this cases if the exposure or possible sources are located within North East and Central London o Initial report is received by the HPU: the information will be relayed to the appropriate EHDs by the HPU o Initial report is received by the EHDs: the information will be relayed to the appropriate EHDs by the EHD receiving the report and also to the HPU. If the exposure or possible sources are located outside North East and Central London: o Initial report is received by NENCL HPU the information will be passed to the appropriate HPU o Initial report is received by EHD in the sector details will be passed to NECL HPU for referral to the appropriate HPU and subsequently the appropriate EHD. The EHD receiving the notification first, might alert all the appropriate colleagues. However, if this is not done it should be discussed with the HPU and a communication strategy agreed to ensure all relevant organisations are informed. Note that this only reflects the pathway of communication between different departments, for urgent cases all departments involved in the investigation should be aware of all the different lines of investigation. For urgent cases there is likely to be a questionnaire that will enclose all this information and that should be shared between all the departments involved e.g. E. coli O157, enteric fever, Legionella 8. COMMUNICATION OF SITUATIONS WHERE CONTAMINATION IS IDENTIFIED Both departments should ensure prompt communication in any situation or incident where exposure to chemicals or radiation is identified. Even in situations where the public health implications are not considered to be significant both departments should alert each other. This could be done via Page 8 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 email if the situation is not assessed as urgent or not needing further actions. Emails to the HPU should be sent to the team email as below: necl.team@hpa.org.uk As the on call team changes on a daily basis; to ensure that communications are not missed EHO should add the team email address when they communicate a new situation to the unit. Otherwise they can communicate using the office number and by talking to a member of the on call team. Page 9 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Table 1: list of organisms and diseases, communication and organisation leading investigation for sporadic cases Disease Acute encephalitis Acute meningitis (viral & bacterial) Acute poliomyelitis Acute Hepatitis A & E Organism Neisseria meningitidis, Sterptococcus pneumonia, Haemophilus Influenzae Polio virus Hepatitis A & E virus Communication between HPU & EHD Actions lead by Weekly report Weekly report HPU action only HPU action only Weekly report Same day HPU action only HPU lead, discuss with EHD to agree investigation and control as necessary HPU action only (unless implications for EHD) Acute Hepatitis B & C & Delta Anthrax Hepatitis B, C & Delta Bacillus anthracis Weekly report (unless actions for EHD) Same day Botulism Clostridium botulinum Routine Brucellosis Brucella spp (Same dayif UK acquired) Chikungunya Cholera Cryptosporidiosis Diphtheria Chikungunya virus Vibrio cholerae Cryptosporidium spp Corynobacterium diphtheriae Corynobacterium ulcerans Salmonella typhoid Salmonella paratyphoid Bacillus cereus Campylobacter spp, clostridium perfringes, Staphylococcus aureus (enterotoxin- strains) Norovirus, Scrombotoxin Same day(if UK acquired otherwise routine) Weekly report Same day Routine Weekly report Enteric fever (typhoid or paratyphoid fever) Food Poisoning (any disease of infectious or toxic nature caused by, or thought to be caused by consumption of food or water) Same day Same day HPU lead, discuss with EHD to agree investigation and control as necessary HPU lead, discuss with EHD to agree investigation and control as necessary HPU lead, discuss with EHO to agree investigation and control as necessary HPU action only HPU lead EHO lead, discuss with HPU HPU action only HPU lead, discuss with EHD to agree investigation and control as necessary EHO lead, discuss with HPU to agree investigation and control as necessary Page 10 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Disease Organism Giardiasis Glander & mieloidosis Giardia Lamblia Burkholderia mallei Burkholderia pseudomallei Streptococcal pyogenes Invasive group A streptococcal disease & scarlet fever Communication between HPU & EHD Routine Weekly report Actions lead by Weekly report HPU action only Same day HPU lead, discuss with EHD to agree investigation and control as necessary HPU action only EHD lead, discuss with HPU to agree investigation and control as necessary EHD lead HPU action only Legionella Legionella spp Leprosy Leptospirosis Leptospira interrogans Listeria Listeria monocytogenes Weekly report Weekly report (unless EHO involvement is needed) Same day Lyme disease Borrelia spp Weekly report Malaria Weekly report Weekly report HPU action only Meningococcal disease Plague Plasmodium falciparum, vivax, ovale, malariae and knowlesi Measles virus, Mumps virus and Rubella virus Neisseria meningitidis Yersinia pestis HPU lead, discuss with EHD to agree investigation and control as necessary HPU lead, discuss with EHD to agree investigation and control as necessary HPU action only Weekly report Same day Psittacosis Q-fever Rabies Chlamydophila psittaci Coxiella burnetii Rabies virus Weekly report Weekly report Weekly report HPU action only HPU lead, discuss with EHD to agree investigation and control as necessary HPU action only HPU action only HPU lead, discuss with EHD to agree investigation and control as necessary Measles, Mumps, Rubella Page 11 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Disease Organism Communication between HPU & EHD Weekly report Actions lead by Relapsing fever Borrelia spp Salmonellosis (not typhoid or paratyphoid) Sever acute respiratory syndrome (SARS) Shigellosis Salmonella spp (not typhoid or paratyphoid) Coronavirus Routine EHO lead Weekly report HPU action only Shigella sonnei Routine EHD lead S. boydii, S. dysenteriae, and S.flexneri Same day HPU lead, discuss with EHD to agree investigation and control as necessary Smallpox Taenia solium (worms) Tetanus TB (note only incidents would be reported) Typhus Viral haemorrhagic fever Variola virus Taenia solium (worms) Clostridium tetani Same day Same day Weekly report Weekly report HPU action only EHO lead HPU action only HPU action only Rickettsia spp Crimean-Congo haemorrhagic fever virus, Guanarito virus, Hanta virus, Junin virus, Kyasanur Forest disease virus, Lassa virus, Machupo virus Marburg virus, Omsk haemorrhagic fever virus, Rift Valley fever virus and Sabia virus Weekly report Same day HPU action only HPU lead, discuss with EHD to agree investigation and control as necessary VTEC including O157 Verocytotoxigenic Escherichia coli (VTEC) including E. coli O157 Bordetella pertussis Yellow fever virus Same day if E. coli O157 Weekly report Weekly report HPU lead, discuss with EHD to agree investigation and control as necessary HPU action only HPU action only Whooping Cough Yellow Fever HPU action only Page 12 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Table 2: Notifiable syndromes or clinical presentations, communication with EHDs and organisation leading for sporadic cases Notifiable syndromes or clinical presentations Communication between HPU & EHD Actions lead by Haemolytic uraemic syndrome (HUS) Weekly report (Same day only if actions for EHO identified) HPU lead, discuss with EHD to agree investigation and control as necessary Infectious bloody diarrhoea Weekly report (Same day only if actions for EHO identified) HPU Lead, discuss with EHD to agree investigation and control as necessary Table 3: Notification of contamination incidents, communication between departments and organisation leading initial response Notification of contamination Communication between HPU & EHD Actions lead by Exposure to chemicals Same day HPU lead, discuss with EHD to agree investigation and control as necessary (depending on the incident it might be decided for EHD to lead the investigation) Exposure to radiation Same day HPU Lead, discuss with EHD to agree investigation and control as necessary (depending on the incident it might be decided for EHD to lead to lead the investigation) Communication definitions Same day: communications with EHO should be on the same day the case is notified. Routine: will be communicated next working day, via encrypted email as it will contain patient identifiable information (PII). Weekly report: will include all cases and incidents reported to and managed by the HPU on-call desk for which EH investigations are not necessarily required e.g. acute encephalitis, meningitis. This report will not contain PII. Note that cases in this report requiring actions from the EHD would have been already reported via the two methods explained above. Page 13 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 9. MANAGEMENT OF ROUTINE SPORADIC CASES 9.1 INTRODUCTION Most of this section deals with routine work considerations, although the principles apply for dealing with any infectious disease cases. Conditions covered include: Food Poisoning (notified without a specific cause) Gastro-enteritis including: o Campylobacter o Giardia lamblia o Salmonella (not Typhoid, Paratyphoid) o Shigella sonnei o Viral gastro-enteritis, e.g. Norovirus o Cryptosporidium Table 5 indicates the agreed minimum standards for investigation of routine single cases. 9.2 RISK ASSESSMENT Public health risk is assessed in terms of continued risk to others. This is in addition to the health of the individual case. There are four specific groups of persons in whom it is particularly important to assess their risk of spreading infection and for whom special action should be considered. Groups that pose an increased risk of spreading infection It is particularly important to assess infected people who belong to one of the four groups for whom special action should be considered. Table 4 Risk groups Group A Any person of doubtful personal hygiene or with unsatisfactory toilet, hand washing or hand drying facilities at home, work or school. Group B Children who attend pre-school groups or nursery. Group C People whose work involves preparing or serving unwrapped foods not subjected to further heating. Group D Clinical and social care staff who have direct contact with highly susceptible patients or persons in whom a gastrointestinal infection would have particularly serious consequences. However, the risk assessment should not only be confined to determine whether cases fit into one of these groups but be based on the organism, the overall lifestyle Page 14 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 of the cases and their contacts, and the severity of the threat. Risk assessment will always be a judgement call and requires knowledge and expertise. People who do NOT pose an increased risk ALL cases of gastroenteritis should be regarded as potentially infectious and should normally be excluded, from work, school or other institutional settings, and advised to refrain from food preparation for others at least until 48 hours after the person is free from diarrhoea and/or vomiting. For some gastrointestinal infections, as detailed in the additional policies and CDR Guidance, microbiological clearance may also be required. 9.3 MANAGEMENT OF SPORADIC CASES OF CAMPYLOBACTER AND SALMONELLA SPP NON TYPHOID OR PARATYPHOID A review of the management of sporadic cases of Campylobacter took place in 2011 in order to evaluate the outcome of the investigation of sporadic cases of this infection. The review followed on a project done by the Regional Epidemiology Unit to evaluate the delay from clinical diagnosis to public health investigation of the cases of Salmonella spp not Typhoid or Paratyphoid. Based on the findings of both reviews, as entered below: - significant delay from onset to public health investigations taking place was observed, reducing the chances of obtaining meaningful information from the case (from 24 days from onset for a lab notification and 13 from onset for clinical notifications) - very poor response to postal questionnaires, better response to telephone questionnaires but more resource intensive - no evidence of any missed outbreaks - anecdotal evidence that outbreaks tend to be identified via the following routes and not by investigating sporadic cases: o members of the public o reports from clinicians It was agreed in the EHO Forum that EHD were not required to follow every sporadic case of Campylobacter and Salmonella spp not Typhoid or Paratyphoid. If they were to do it a telephone questionnaire would be the indicated way. However, this will be a resource intensive action with uncertain value due to the delay in reporting. It was agreed that it would be more useful to deploy resources to the investigation of these cases only when significant increases occurred in a particular area or particular time that may appear suspicious. The Regional Epidemiology Unit will review cases in the sector on a weekly basis and alert the unit when increases above the expected levels are identified. The Unit will communicate with the relevant EHDs to discuss and agreed if these findings need to be investigated further. Page 15 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 9.4 MINIMUM AGREED ACTIONS Table 5 Minimum agreed actions Minimum EH/HPU Procedures following notifications of potential Food-borne Pathogens For single cases (not outbreak or high risk group situations) Hepatitis A* Contact ASAP (Visit or telephone and/or clarification with clinician) Initial contact – may be conducted by HPU or EHD HPU Aeromonas EHO Bacillus cereus / subtilis EHO Campylobacter*** EHO Clostridium perfringens EHO Cryptosporidium E coli O157* EHO 1st HPU 2ndEHO E coli non-VTEC EHO Food poisoning non-specific Giardia Salmonella nonTyphoid or Paratyphoid*** Send appropriate fact sheet / advice** Yes (food poisoning fact sheet) (food poisoning fact sheet) No need for sporadic cases Yes (food poisoning fact sheet) Yes Yes Yes (food poisoning fact sheet) Attempt to clarify by sending a letter with questionnaire Clearance samples required for cases and/or contacts See additional guidance NO NO NO No need for sporadic cases NO NO NO NO EHO Yes NO EHO Yes NO EHO No need for sporadic cases No need for sporadic cases NO Shigella sonnei EHO Yes NO Shigella non-sonnei * Typhoid & Paratyphoid fever* Taenia solium (worms) 1st HPU 2nd EHO Yes √ √ 1st HPU 2nd EHO Yes Yes (link to NHS x information) Yes (food Vibrio non1st HPU poisoning fact NO cholera 2nd EHO sheet) Yes (norovirus or Viral EHO rotavirus fact NO gastroenteritis sheet) NO Yersiniosis HPU Yes *These notifications are urgent due to its public health implications. Therefore, all possible efforts should be made to contact the case or relatives ASAP. If this is not possible by phone a home visit by the EHO should be organised at the earliest opportunity. ** Copies of the factsheets and links can be found in appendix 1c *** No need to investigate sporadic cases, only to be investigated if increases are identified by the Regional Epidemiology Unit (refer to page 15, 9.3 for further information) EHO Page 16 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 9.5 FURTHER INVESTIGATION Note that in outbreak or cluster investigations further information is to be gathered this should be based on the General Food and Environmental Questionnaire (Appendix 1c). Please refer to the appropriate policy for more targeted questionnaires for specific conditions. The questionnaires for specific conditions, s.typhi/paratyphi, VTEC O157 and Hepatitis A, etc will need to be shared between HPU and the EHD. For investigations link to particular events a more targeted questionnaire might need to be developed. Both departments should discuss the development of more targeted questionnaires. A final questionnaire will need to be agreed by both parts. For outbreak situations this discussion is likely to take place during an outbreak control meeting. 9.6 SPECIMEN COLLECTION Specimens of stool and vomit form the cornerstone of human gastrointestinal disease investigation. However, these are only part of the range of investigations which should be considered. Environment and food samples and NHS samples of blood and other clinical sites may also be appropriate. Stool tests are not generally required from suspected routine simple cases e.g Campylobacter, unless there are concerns about an outbreak or as part of a wider investigation. Vomit samples should only be submitted after discussion with your local consultant microbiologist. To get the best possible specimens people should be given the following: A brief explanation of why samples are needed, how many and at what intervals. The correct specimen pots and laboratory request forms. Clear written instructions on how to obtain samples (suitable for people to follow themselves or for care workers to follow). See Fact Sheet File for fact sheets and guidelines. Clear instructions as to when samples will be collected or where they can be delivered. Ideally, samples should be collected by the EHO. Barts and the London is the HPA laboratory. The laboratory will provide public health microbiology services for the Health Protection Units, Environmental Health Officers (EHOs) and Primary Care Groups across London. This will include the examination of clinical specimens submitted for the investigation of suspected and confirmed outbreaks and other incidents of public health importance, excluding those occurring in hospitals. It is important to note that the Barts and The London will primarily process clinical samples, while food, water and environmental specimens should be sent directly to the HPA Food Water & Environmental Laboratory at Colindale. Page 17 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Each sector has a named Consultant Microbiologist. They are the first point of call for all incidents and outbreaks and will be available for advice and direction during working hours. Further information on microbiology arrangements can be found in Health Protection Agency Barts and The London, HPA Laboratory Guidebook for Environmental Health Officers, Consultants in Communicable Disease Control and Primary Care Groups (Appendix 1c) Microbiologist for North Central London will provide advice to the following boroughs: North Central London including Kensington, Chelsea, Westminster, Camden, Islington, Barnet and Haringey Microbiologist for North East London will provide advice to the following boroughs: North East London including City of London, Hackney, Tower Hamlets, Waltham Forest, Barking and Dagenham, Havering, Redbridge and Newham 9.7 CONTROL 9.7.1 General advice The circumstances of each case, excreter, carrier or contact in a risk group should be considered individually. Amendments to the Health Protection Regulations in 2010 require a change in the way that cases or contacts are advised and requested to remain away from work/school/nursery for a period of time if needed. The HPU may only “advise” cases/contacts to remain away from work/school/ nursery or specific activities such as food handling for a period of time as recommended by policy and guidance in conjunction with individual risk assessment: Advise them of the need to remain away in order to prevent possible onward transmission, Advise them to inform their workplace/school/nursery of this advice, Give information/fact sheet, Advise them that EHO will follow-up next day Inform EHO that this information/advice has been given so they can follow-up next day as necessary The local authority (usually EHO) may “request” that cases remain away from work/school nursery or specific activities for a period of time and may instigate appropriate legal action as necessary if requests are not complied with. Often formal legal action may not be necessary since unwell patients are able to remain off work either through self-certification or a certificate from their GP. On Page 18 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 occasion (sometimes in respect of carriers of organisms who are otherwise well) it may be decided by the LA and HPU that a lengthy absence from work/school/nursery or a specific activity is needed, in which case the LA may be required to pay compensation. Implicated cases or contacts will need to discuss this directly with the LA involved to explore options available. With the exception of typhoid, paratyphoid and infections caused by Verocytoxin producing E.coli (VTEC) O157, shigella dysenteriae, flexneri & boydii, negative stool samples are not necessary for persons returning to work after a diarrhoeal illness. See section 10 below, which deals with infections requiring urgent attention. Some operators of food processes may have their own food handling policies which cover these situations. 9.7.2 General advice in relation to gastrointestinal disease Initially all those who are symptomatic should be advised to stay away from their normal daily activities until 48 hours after they have recovered clinically (but ensuring that this follows stopping any medication, such as Imodium, which might be masking symptoms). The final decision about returning is affected by the type of work or other activity and by the organism isolated. Advice will be provided to the case and contacts as relevant either through conversation when initially contacting the patient and posting information leaflet as per Table 5. 10. DEALING WITH SINGLE URGENT CASES When cases of E.coli (VTEC), S.typhi, S.paratyphi, hepatitis A, and shigella (excluding s.sonnei) are reported there is a need to take immediate action to ensure the appropriate public health actions can be instigated i.e. assessment and exclusion of cases and contacts and arrangement for immunisation of contacts as appropriate (see below for hyperlinks). The response to such cases must therefore be immediate. This will often require prompt liaison between the EHDs and HPU as the routes of notification differ i.e. via GPs, Micro, managers etc. Prompt communication will ensure that an early risk assessment can be conducted and public health risks can be reduced. This will also ensure that there is a co-ordinated response. As specialist assessment is required to be undertaken by the EHD and the HPU it is essential that notification of these cases is communicated early. For example the response to a case of hepatitis A will require prompt assessment of contacts requiring immunisation and/or human immunoglobulin as well as identification of contacts at risk (i.e. in schools, nurseries etc) which will need to assess as early as possible. Page 19 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 For conditions not covered in this document or when there is any uncertainty both Departments should consult each other as a matter of urgency to determine whether further action is required and agree roles and responsibilities. Some notifiable diseases, as outlined in Table 1 as the ones that need to be communicated on the same day, could constitute emergency conditions with National implications (e.g. Viral Haemorrhagic fever). If these conditions fall within the remit of joint working between the EHDs and HPU team then discussion between the two departments should happen as early as possible. These conditions include: Potentially complicated gastrointestinal disease, due to risk assessment required for cases and contacts e.g. VTEC; typhoid and paratyphoid fever, hepatitis A, shigella and cholera. Please refer to the specific policies below Other conditions with food or environmental related transmissions such as leptospirosis and legionnaire’s Disease Other notified conditions are mainly the remit of clinicians and the HPU team. Those requiring communication to the HPU by phone or fax on the same day include: measles; mumps; rubella; scarlet fever and whooping cough or through written notification reporting e.g. malaria. The following diseases are considered urgent. Therefore, there are specific policies for them, which should be referred to: o Hepatitis A o Legionella o Listeria monocytogenes o Salmonella typhoid and paratyphoid o Shigella non sonnei o VTEC O157 (supporting evidence) Copies of the policies above can be found in Appendix 1c. The policies and guidance in appendix 1c are the most current ones at the time when this document was finalised in September 2011. If in any doubt the EHDs should liaise with the HPU to ensure they have the latest versions. The HPU will communicate any changes in policies or guidance via the EHO forums and will use the mailing list of the forum to cascade any relevant information regarding changes to the policies or guidance. Page 20 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 11. REFERENCES o Working Group of the former PHLS Advisory Committee on Gastrointestinal Infections 2004 Preventing person-to-person spread following gastrointestinal infections: guidelines for public health physicians and environmental health officers. Commun Dis Public Health 2004; 7(4): 362-384 o The Health Protection (Notification) Regulations 2010; http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@ ps/documents/digitalasset/dh_114589.pdf o Hawker J, Begg N, Blair I, Reintjes R, Weinberg J 2005 Communicable Disease Control Handbook. (2nd edition) Blackwell, London o Guidance on infection control in schools and other childcare settings, Health Protection Agency, April 2010 o Guidance on Infection Control and Communicable Diseases in Schools, Colleges and other childcare settings; North East and North Central HPU, May 2010 Page 21 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Appendix 1a – Disease Information Notifiable Disease Information This section includes brief notes providing disease information. For gastrointestinal disease the information has been taken from the 2004 Working Group Advisory Committee on Gastrointestinal Infections (of the former PHLS ). For other notifiable diseases the information has been compiled from Heyman (2005) (Control of Communicable Disease Manual), Hawker et al (2005), Communicable Disease Control Handbook, and the HPA web site. This section also includes details on chemical food-borne illness. This information has been taken from the Department of Health 1994 publication on the management of outbreaks of food-borne illness. Note that the diseases, for which a specific policy is in place, might not appear in this appendix. Further information on these diseases can be found in the embedded documents in section 8.1. See reference section (page 17-18) for full details. Table 1 – Clinical features of gastrointestinal infections, infestations and intoxications Causative agent Aeromonas sp Bacillus cereus emetic syndrome diarrhoeal syndrome Bacillus subtilis group B. subtilis B. licheniformis Campylobacter sp Cholera Clostridium botulinum Clostridium perfringens Cryptosporidium sp Dysentery amoebic Escherichia coli (ETEC) (EPEC) Incubation period Common clinical features Vomiting, diarrhoea Mode of transmission Water and contaminated food 1 to 6 hours Nausea, vomiting & abdo pain Diarrhoea, abdominal pain Ingestion of contaminated food 10 minutes to 14 hours 2 to 14 hours 1 to 10 days(usually 2 to 5 days) a few hours to 5 days (usually 2 to 3 days) 2 hours to 8 days, (commonly 12 to 36 hrs) 4 to 24 hours (usually 12 to 18 hours) 1 to 14 days Nausea, vomiting, diarrhoea Diarrhoea, abdominal pain Abdominal pain, profuse diarrhoea malaise; vomiting is uncommon Profuse watery diarrhoea rapid dehydration Ingestion of contaminated food Dysphonia, diplopia, dysphagia ptosis Ingestion of contaminated food Diarrhoea and abdominal pain Ingestion of contaminated food Watery or mucoid diarrhoea Usually 2 to 4 weeks 10 to 72 hours 9 t0 12 hours Bloody diarrhoea Faecal oral, contaminated water, animal contact Faecal oral 6 to 24 hours Diarrhoea Ingestion or handling of contaminated food or water Contaminated food or water Faecal oral, occasionally food or water Page 22 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Causative agent Incubation period Common clinical features Mode of transmission Escherichia coli (VTEC) 1 to 8 days Ingestion of contaminated food, faecal oral Giardia 3 to 25 days Haemorrhagic colitis haemolytic uraemic syndrome Diarrhoea, abdominal cramps Hepatitis A virus 15 to 50 days Fever, malaise, nausea, jaundice Listeriosis 1 day to >3 months Septicaemia, meningitis, influenza like illness Salmonella Typhi Paratyphi 1 to 3 weeks 1 to 10 days Salmonellas (nonenteric fever) 12 to 72 hours (usually 12 – 36 hours) 12hrs to 7 days Fever, malaise, nausea, constipation (early), diarrhoea (late) Diarrhoea, vomiting, and fever Shigella sp Staphylococcus aureus Vibrios (noncholera) eg V. parahaemolyticus Viral gastroenteritis Norovirus Rotavirus Worms Yersinia sp 1 to 7 hours (usually 2 to 4 hours) 2 to 48 hours (usually 12 to 18 hours) usually 10 to 50 hours Ingestion of contaminated food or water S. sonnei – generally mild Bloody diarrhoea Other species more severe Vomiting, abdominal pain Faecal oral, occasionally contaminated food or water Ingestion of contaminated food Diarrhoea, fever Ingestion of contaminated food particularly shellfish Vomiting predominates, diarrhoea, fever Diarrhoea, vomiting From cases (including 48 hrs post recovery) Faecally-orally by ingestion inhalation of vomit and fomites Wide range (see text) Faecal oral Watery diarrhoea, abdominal pain fever, arthritis Ingestion of contaminated food 24-72 hrs Life cycle dependant 3 to 7 days Faecal oral; ingestion of contaminated food or water Faecal oral; ingestion of contaminated food or water Contaminated food or vertical transmission or rarely HCAI Ingestion of contaminated food or water Page 23 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Acute Encephalitis Control of human source Statutorily notifiable Cases Dependent on cause. Expert infectious disease advice may be needed. Contacts/exposed Unlikely that contacts will require any intervention unless a particular virus can be transmitted from person to person. Isolation/exclusion Dependent on cause Cause There are many types of encephalitis, most of which are caused by viral infection. Reservoir Human and a wide variety of animals can carry viruses associated with encephalitis. Many are outside the UK. Transmission. Various vectors including mosquitoes, ticks, and a wide variety of animals. Other relevant features Usually notified as part of viral meningitis. May be associated with long-term neurological diseases. Just after the first World War, a viral disease, encephalitis lethargica, attacked almost 5 million people throughout the world, and then suddenly disappeared in the 1920s. Known as sleeping sickness in the United States, this disease killed one third of its victims and in many others led to post-encephalitic parkinsonism Page 24 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Acute Poliomyelitis Control of human source Statutorily notifiable Cause Poliovirus Cases Isolation and enteric precautions Reservoir Humans Contacts/exposed Immunisation Transmission. Person to person faecal-oral route Isolation/exclusion Isolation of contacts required Other relevant features Although no cases in UK in recent years still endemic in many countries of the world. An acute case in this country would trigger a major incident. Aeromonas Control of human source Statutorily notifiable if thought to be the cause of food poisoning. Person to person spread is rare. Cases Enteric precautions. Contacts Clinical surveillance only. Exclusions Cases in risk groups A to D for 48 hours after first normal stool. Microbiological clearance None required Cause Aeromonas hydrophila, A. sobria or other members of the group. Reservoir Common free-living organisms in aquatic environments. Transmission Consumption of untreated water, contaminated shellfish, and foods eaten raw or washed in untreated water. Other relevant features Pathogenicity of many strains is unproven. Some strains produce a toxin like that of Vibrio cholerae. Page 25 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Amoebic Dysentery Control of human source Dysentery is statutorily notifiable. Cause Entamoeba histolytica. Cases Enteric precautions until treatment is completed. Reservoir Human gastrointestinal tract. Contacts Screen household contacts. Microbiologically to detect cyst excreters. Exclusions 48 hours after first normal stool. C and D require microbiological clearance. Transmission Waterborne or via contaminated raw or undercooked foods. Transmission is usually through faecal oral spread from a chronically ill or asymptomatic cyst shedder. Other relevant features Cysts resist standard chlorination but are destroyed by hyperchlorination or iodination. Microbiological clearance Cases in risk groups C and D: One stool, obtained at least one week after the END of treatment, should be examined for E. histolytica cysts. Careful assessment of excreters is needed to evaluate significance because pathogenic E. histolytica cysts are morphologically indistinguishable from those of the non-pathogenic E.dispar. Referral of specimens for cyst typing can be undertaken. Page 26 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Anthrax Control of human source Statutorily notifiable. A possible association with bioterrorism (CBRN). Cause Anthrax is a bacterial infection caused by Bacillus anthracis. Cases Cases do not require isolation after they have been decontaminated. Reservoir The spores produced by the bacterium may survive for periods up to 30 years or longer and are resistant to desiccation. Contacts/exposed Contacts of anthrax spores as a result of CBRN need decontamination. Antibiotic prophylaxis and immunization may be needed. Isolation/exclusion Contacts should be isolated until decontaminated. If Anthrax is considered as part of CBRN major incident plans should be activated. Transmission. Anthrax is primarily a disease of animals not humans. It is extremely unusual for anthrax to be transmitted from person to person. Transmission is by contact with infected animals, contaminated hides, wool or products made from these or contact with soil associated with infected animals or contaminated bone meal or through deliberate release as in bioterrorism. Other relevant features Incubation period is 1 - 7 days, although can be up to 60 days. Page 27 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Bacillus species food poisoning Control of human source Statutorily notifiable as food poisoning. Person-to-person spread does not occur. Cause 1. Bacillus cereus. 2. Bacillus subtilis group, including B. licheniformis. Cases Enteric precautions. Reservoir No human or animal sources. Environment, soil, sediments, dust, vegetation. Food: cereal products herbs and spices, dried foods, milk and dairy products, meat and meat products. Contacts No action necessary. Exclusions 48 hours after first normal stool. Microbiological clearance None required. Transmission Contaminated cooked foods subjected to inadequate post-cooking temperature control during cooling and storage. B.cereus: mainly rice dishes; occasionally pasta, meat or vegetable dishes, dairy products, soups, sauces, sweet pastries. B.subtilis group: mainly meat or vegetable with pastry products, cooked meat or poultry products, occasionally bakery products, including bread, crumpets, sandwiches, and ethnic meat or seafood dishes. Other relevant features B.cereus causes two distinct clinical presentations – the emetic and diarrhoeal syndromes – that are associated with different toxins. Page 28 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Campylobacter Control of human source Statutorily notifiable as food poisoning if thought to be foodborne or waterborne. Cases Enteric precautions. Contacts Clinical surveillance. Exclusions 48 hours after first normal stool. Microbiological clearance None required. Cause Predominantly Campylobacter jejuni. C. coli and other Campylobacter species (eg C. lari and C. fetus) are much less frequently implicated. C. jejuni predominantly causes enteric illness (acute gastroenteritis, colitis),while C. fetus is the major pathogen in extraintestinal illness (systemic illness with bacteraemia, meningitis, vascular infection, abscesses, occasionally gastroenteritis). Reservoir Campylobacter infection is a zoonosis. The reservoir for C. jejuni is very varied and includes the gastrointestinal tract of birds (particularly poultry) and mammals (i.e. cattle and domestic pets). C.coli is most commonly isolated from pigs, and C. fetus from cattle and sheep. Transmission Transmission is predominantly via contaminated food or water. Person-to-person transmission can occur if hygiene is poor Other relevant features Not all Campylobacter infections result in illness. The infective dose is considered to be low. Person-to-person transmission can occur and school age children can rarely transmit campylobacter infection. Occupational exposure has been implicated in some illness. Campylobacter is a fairly frequent cause of travellers’ diarrhoea. Campylobacter does not multiply on food and foodborne disease outbreaks are rarely recognised. In a recent review of outbreaks the most frequently identified food-handling fault was crosscontamination. Transmission from ill food handlers is extremely rare. Page 29 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Cholera Control of human source Statutorily notifiable. Cases Enteric precautions. Cases should normally be admitted to an Infectious Diseases Unit. Contacts Clinical surveillance of people who shared food and drink with case for 5 days from shared exposure. If secondary transmission is likely, chemoprophylaxis with tetracycline, doxycycline or erythromycin. Exclusions 48 hours after first normal stool. Microbiological clearance Where indicated, two consecutive negative stools taken at intervals of at least 24 hours apart. Cause Vibrio cholerae O1 (biotypes classical and El Tor) and V. cholerae O139. Clinical disease is mediated by the production of an enterotoxin. Reservoir The natural reservoir is aquatic environments. Vibrio cholerae lives attached to a special sort of algae or to crustacean shells and zooplankton. In favourable environmental conditions V. cholerae survives for years in a free-living cycle without human intervention. When growth and survival conditions are sub-optimal, V. cholerae switches to a viable, non-culturable state. Although humans infected with V. cholerae may shed organisms for prolonged periods, their importance as a reservoir is trivial compared with the aquatic environment. Transmission Transmission is predominantly through consumption of polluted untreated water, contaminated shellfish and raw food or food washed in contaminated waters. V. cholerae has been shown to survive for up to 14 days in some foods, particularly when the food was contaminated post preparation. Symptomatic and asymptomatic food handlers have been implicated in foodborne outbreaks. Although there are reports of person-to-person transmission in the literature, it has been suggested that other potential risk factors might have been overlooked in disease transmission in some of these outbreaks. Other relevant features Cholera is rare in the United Kingdom (UK) and only occurs in imported cases. For person-toperson transmission to occur a large inoculum is required. Vaccination plays no part in the management of contacts or the control of outbreaks. Page 30 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Clostridium botulinum – botulism Control of human source Statutorily notifiable as food poisoning. Cause Clostridium botulinum neurotoxin. Person-to-person spread does not occur. Reservoir Soil and aquatic sediments. Cases Hospital admission imperative. Immediate investigation with associated laboratory studies to identify the source. No other control measures are required for the case. Others exposed to the same source Immediate identification and treatment of others at risk is mandatory. Examination of faecal and serum specimens of people exposed may be indicated. Contacts Only important as potential cases if they have been exposed to the same risk of infection. Exclusions None. Microbiological clearance None required. Transmission Foodborne botulism in adults is generally caused by ingestion of food contaminated with toxin. Under processed non-acid foods or foods contaminated after canning or bottling (eg canned fish) may provide anaerobic conditions and suitable pH for the organism to multiply during prolonged storage at room temperature. Infant botulism is caused by ingestion of spores. Wound botulism can also occur when spores get into the body via a contaminated wound. Other relevant features Foodborne botulism is rare in the UK, but it is a severe disease with a high mortality rate. A single case, especially if caused by eating a commercially produced food, may signal a national emergency and prompt action is essential at any time of the day or night. The local microbiologist and CCDC should be contacted urgently, as should HPA Centre for Infections (telephone 020 8200 4400) or HPS (0141 300 1100). Suspect foods and clinical specimens (serum, vomit, faeces) (including from Scotland) should be sent immediately by courier to the HPA Centre for Infections, (telephone 020 8200 4400) for testing. Botulism is ultimately a clinical diagnosis that laboratory tests can confirm but not refute. Once a clinical diagnosis is made botulinum antitoxin must be given as soon as possible. Details of holding centres nationwide are available from the HPA duty doctor. Enforcement officers should refer to the Food Safety Act 1990 – Code of Practice No. 16: enforcement of the Food Safety Act 1990 in relation to the food hazard warning system. Page 31 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Clostridium difficile antibiotic-associated diarrhoea (AAD) Control of human source Cases Enteric precautions. Stop predisposing antibiotic therapy. Initiate appropriate treatment. Contacts Monitor susceptible individuals (elderly, all those receiving antibiotics) for diarrhoeal illness. Exclusions 48 hours after first normal stool. Asymptomatic carriers should not be excluded from nursing homes/ hospitals. Microbiological clearance. None required. Cause Clostridium difficile, which produces toxins damaging to the colonic mucosa. Reservoir Human gastrointestinal tract. Spores survive in the environment around symptomatic cases. Transmission Person-to-person by the faecal-oral route and by environmental contamination. Antibiotic use (especially broad-spectrum agents) disrupts the normal bacterial flora and induces susceptibility to C. difficile colonisation and overgrowth. Other relevant factors AAD ranges in severity from mild diarrhoea to severe pseudomembranous colitis. Outbreaks occur in hospitals and nursing homes. The elderly are particularly susceptible. Young children (aged less than 2 years) can carry C. difficile as part of the normal bowel flora and asymptomatic carriage occurs in a small proportion of adults. Page 32 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Clostridium perfringens food poisoning Control of human source Statutorily notifiable as food poisoning. Person-to-person spread does not occur. Cases Enteric precautions. Contacts No action necessary. Exclusions 48 hours after first normal stool. Microbiological clearance None required. Cause Clostridium perfringens enterotoxin. Reservoir Gastrointestinal tract of food animals, soil and dust. Transmission Contaminated cooked meat and poultry dishes subjected to inadequate temperature control after cooking or cooling, inadequate reheating before consumption. Other relevant features C. perfringens enterotoxin is produced in the intestine after ingestion of large numbers (Usually >105) of organisms. The toxin is produced when the ingested organisms transform into spores in the intestine. Page 33 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Cryptosporidiosis Control of human source Statutorily notifiable as food poisoning if thought to be food or waterborne. Cases Enteric precautions. Contacts Clinical surveillance. Exclusions 48 hours after first normal stool. Cases should also avoid using swimming pools for two weeks after the first normal stool. Microbiological clearance None required. Cause Cryptosporidium spp. Reservoir Gastrointestinal tracts of humans and animals. Transmission Direct or indirect contact with infected animals. Person-to-person spread, particularly in households, healthcare and nurseries. Water contaminated directly or indirectly with faeces. Outbreaks have been associated with public and private water supplies, swimming pools and, more rarely, contaminated food. Seasonal outbreaks are associated with farm visits to feed and handle lambs and calves. Other relevant features Oocysts resist standard chlorination. Large numbers of organisms are excreted during acute infection. The infectious dose is low. Since oocysts can continue to be shed following cessation of diarrhoea, it is recommended that cases avoid using swimming pools for normal stool. Immunocompromised individuals are particularly susceptible and may be unable to clear the parasite. Anyone whose T cell function is compromised should be advised to boil and cool their drinking water, and water for making ice, from whatever source. Page 34 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Cyclosporiasis Control of human source Statutorily notifiable as food poisoning. Direct person-to-person transmission is unlikely. Cases Enteric precautions. Treatment with trimethoprim sulphamethoxazole is rapidly effective. Contacts No action necessary. Exclusions 48 hours after first normal stool. Microbiological clearance None required. Cause Cyclospora cayetanensis. Reservoir Human gastrointestinal tract. Transmission Routes of transmission are poorly understood. Outbreaks have been associated with the consumption of soft fruit and vegetables (foods that are difficult to wash and are eaten raw) and drinking water. Other relevant features Direct person-to-person transmission is unlikely since oocysts require a maturation period after shedding and are only infectious once spores are produced. Food handlers could contaminate food for later consumption. Diarrhoea may follow a relapsing and remitting course. Immunocompromised people may be infected for some months but treatment will clear the infection. Page 35 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Diphtheria Control of human source Statutorily notifiable. Diptheria is a public health emergency. The first action is to determine whether it is a toxigenic diphtheria (if it is not then no public health action is required). Cause Toxin carried by some Corynebacterium diphtheriae and some Corynebacterium ulcerans Cases Isolate in hospital Transmission. Contact with a patient or carrier; more rarely, contact with articles soiled with discharges from lesions of infected people. Contacts/exposed Contacts should receive antibiotics. All contacts should be monitored daily for 7 days to ensure they are well. Immunization boosters as recommended. Isolation/exclusion Adult contacts that are food handlers should be excluded from work until found to be culture negative (diphtheria is occasionally transmitted by food and milk). Reservoir Humans Other relevant features Classical respiratory diphtheria is characterised by the insidious onset of membranous pharyngitis with fever appearance. Cutaneous diphtheria usually appears on exposed body parts, especially the legs. The lesions start as vesicles and quickly form small, clearly demarcated, and sometimes multiple ulcers. Page 36 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Escherichia coli – Vero cytotoxin-producing (VTEC) Control of human source Statutorily notifiable if thought to be food poisoning. The informal reporting of haemolytic uraemic syndrome (HUS), particularly with a diarrhoeal prodrome, is to be encouraged. Cases Enteric precautions. Hospital admission if haemorrhagic complications occur. Isolation only during acute diarrhoeal phase. Contacts Contacts in risk groups A to D should be screened microbiologically, initially to identify excreters and subsequently for microbiological clearance (below). Authorities must satisfy themselves of the adequacy of hygiene and toilet facility arrangements. Hand washing by children must be supervised in nurseries and infant schools. Exclusions 48 hours after the first normal stool for cases not in risk groups. Cases and contacts in risk groups A to D until microbiological clearance is obtained. Microbiological clearance Risk groups A to D only – two negative faecal specimens taken at intervals of not less than 24 hours. The ease of spread means that it may be wise to ensure that all cases and contacts in high-risk groups in a given household or similar setting are no longer excreting before being allowed to return to work, school, etc. Such risks depend in part on the risk of transmission in the household and can ultimately only be assessed locally. Cause Vero cytotoxin-producing Escherichia coli. The commonest serotype in the United Kingdom is E.coli O157:H7. Reservoir The gastrointestinal tract of cattle, sheep, goats and other, particularly domesticated, animals. The disease is a zoonosis. Transmission Person-to-person spread can occur by direct contact (faecal-oral), particularly in households, nurseries and infant schools. (In confirmed cases of VTEC infection, younger primary school children, whose ability to practice personal hygiene may be limited, should be managed as risk group B). Evidence for spread from asymptomatic healthcare staff and food handlers remains elusive. Primary infections are acquired by: contact with infected animals or their faeces, particularly on farms, including ‘open’ farms. Contaminated foodstuffs – beef and other meat products (for example, undercooked beef burgers and contaminated cooked meats) and raw dairy products have been associated with cases or outbreaks. Extensive waterborne outbreaks have occurred. Other relevant features VTEC may give rise to a haemorrhagic colitis and about 5% of cases progress to the haemolytic uraemic syndrome, of which the case fatality rate is about 2%. Antibiotic treatment may be harmful. Diagnosis may be made by a serum sample even after microbiological clearance and a salivary sample may be a possible alternative, particularly for younger children. Page 37 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Escherichia coli other than VTEC Control of human source Cases Enteric precautions for EPEC, EAggEC and ETEC. Cases of EPEC admitted to hospital should be isolated if possible. Contacts Clinical surveillance. Exclusions 48 hours after first normal stool. Microbiological clearance None required. Cause Some classical enteropathogenic E.coli (EPEC) belonging to a small number of serotypes cause sporadic cases and outbreaks of diarrhoea in children, usually under the age of 2 years. A wider range of EPEC strains have caused sporadic cases and outbreaks affecting adults. Enterotoxigenic E.coli (ETEC) are a major cause of travellers’ diarrhoea. Enteroaggregative E.coli (EAggEC) cause sporadic cases and outbreaks affecting all ages. Associated with travel abroad. Other types such as enteroinvasive E.coli (EIEC) rarely occur in the UK. Reservoir Human gastrointestinal tract, including ‘classical’ EPEC. Infection with some EPEC strains may be zoonotic. Transmission EPEC in day care units and nurseries transmitted from person-to-person by the faecal oral route. Sporadic cases and outbreaks of non-classical EPEC infection may be caused by contaminated food. ETEC in contaminated food and water. Person-to-person spread is unusual. EAggEC in contaminated food. Page 38 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Food Poisoning Control of human source Statutorily notifiable Cases Investigation if possible. Promotion of good hygiene. Cause Food poisoning may be notified without information on a causative agent. It may be caused by any of the agents within this disease list. Contacts Clinical surveillance only Exclusions Cases in risk groups A to D for 48 hours after first normal stool. Microbiological clearance Not possible if food poisoning with no identifiable cause. Giardiasis Control of human source Statutorily notifiable as food poisoning when believed to be foodborne. Antimicrobial treatment of individual cases forms the basis of control. Cases Enteric precautions. Contacts Screening household contacts may identify excreters who need treatment. Exclusions 48 hours after first normal stool. Microbiological clearance None required. Cause Giardia duodenalis (syn. Giardia lamblia, syn. Giardia intestinalis). Reservoir Human gastrointestinal tracts. Animal hosts have been described but this is seldom the source of disease in humans. Transmission Person-to-person. Faecal-oral route is important in young children. Spread within families is common. Foodborne outbreaks have occurred, particularly linked to infected food handlers or contacts. Water contaminated with faeces. Outbreaks have been associated with drinking and recreational waters. Other relevant features Cysts resist the levels of chlorine normally used in drinking water treatment. Page 39 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Hepatitis A Control of human source Statutorily notifiable as viral hepatitis. Cause Hepatitis A virus (HAV). Cases Enteric precautions. Reservoir Human gastrointestinal tract. Contacts Vaccination of household contacts should be considered if the index case was identified within one week of onset of illness (usually defined by jaundice) or if at continuing risk. Alternatively, passive immunisation with human normal immune globulin to family, sexual, household, and other close contacts should be considered. Hand washing by children must be supervised in nurseries and infant schools. Authorities must satisfy themselves that hygiene and toilet facilities are adequate. Consider active immunisation in selected outbreaks and seek advice. Transmission Person-to-person spread is common by faecaloral route, including through sexual intercourse and probably through urine. Food contaminated by an infected person. Consumption of contaminated untreated water, contaminated shellfish, and foods eaten raw or washed in these waters. Other vehicles have been fresh picked fruits and products such as ice cream made from them. Other relevant features Young children are commonly asymptomatic. Older children and adults usually have symptoms. The period of infectivity starts 10 to 14 days after exposure and a similar interval before the onset of jaundice but is maximal just before jaundice develops. Specific HAV IgM is Others exposed People who have recently been exposed to diagnostic but not detected until 5 days after food prepared by a case may benefit from onset of symptoms. Susceptibility may be active or passive immunisation. determined by the examination of a blood or saliva specimen for anti-HAV IgG. Bloodborne transmission is described but rare, Exclusions All cases including those in risk groups A associated with injecting drug use or blood to D should be excluded for 7 days after products. onset of jaundice and/ or other symptoms. Microbiological clearance None required. Page 40 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Hepatitis E Control of human source Statutorily notifiable as viral hepatitis. Cause Hepatitis E Virus Cases Enteric precautions Reservoir Gastrointestinal tract (human and animals) Contacts Although Hepatitis E infection usually produces only mild disease. However, when hepatitis occurs in pregnant women, there may be a case fatality of up to 20% in the third trimester. Transmission Especially water contaminated by an infected person/animal. Food borne infection also occurs. Secondary transmission low (1-2%). Exclusions For all cases and those in Risk Groups up to 14 days after first experiencing symptoms. Advise to avoid contact with pregnant women. Other relevant features Hepatitis E has historically been seen as a disease associated with travel to countries where it occurs more commonly in the population, including the Indian subcontinent, Africa and central America. Most clinically reported cases occur in young or middle aged adults. Microbiological clearance None required Legionnaire’s disease Control of human source Legionnaire’s disease is a notifiable disease. Cases Isolation not required Contacts / exposed Administer Legionnaire’s disease questionnaire. Cause Bacteria - Legionella pneumophila Reservoir Ubiquitous – widely distributed in soil and water. Particularly in water-cooled air conditioning systems and cooling towers. Transmission Mode of transmission – waterborne, thought to be mainly aerosol. Not person to person. Isolation / exclusion Not required Other relevant features Patients are often severely ill. Pontiac fever is a milder form of the disease without pneumonia. Incubation period is 5-66 hours for Pontiac fever and 2-10 days for Legionnaire’s disease. Page 41 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Leprosy Control of human source It is a statutorily notifiable disease Cause Mycobacterium leprae Cases None except lepromatous leprosy. None for chronic cases. Reservoir Humans Contacts / exposed Clinical surveillance Transmission. Close personal contact Other relevant features Isolation / exclusion Assistance of DPH and/or EH may be requested in locating family and household contacts for examination. Contracted outside UK The HPU team receives an annual request for information about known cases of leprosy. Leptospirosis Control of human source Statutorily notifiable as a causative agent. Cases Isolation not required Contacts / exposed No action required Environmental control Ensure contamination of domestic environment by urine of potentially infected animals is avoided. Ensure rodent control adequate. If rodents a particular problem consider ways to reduce their population and make the environment unsuitable for them. Eliminate potential contamination or prohibit use of water Isolation / exclusion Not required Cause Spirochaete Leptospira hardjo (cattle) & Leptospira icterohaemorrhagiae (rats) Reservoir Wild and domestic animals, rats, pigs, cattle, dogs, badgers. Spirochaetes may be present in the urine after recovery. Transmission. Through direct or indirect (the majority) contact with infected animal urine, or, less frequently, from animal bites, handling infected animal tissues or swallowing contaminated food or water. Person to person spread is exceptionally rare. The bacteria enter through skin abrasions or through the eyes nose and mouth. Other relevant features Weil’s disease – may lead to renal failure. Vaccine is only licensed for veterinary use. Page 42 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Listeriosis Control of human source Treatment of individual cases and removal of contaminated foods. Statutorily notifiable as food poisoning. Person to person spread does not occur except during the neonatal period. Cases Admission to hospital and treatment with antimicrobial agents Cause Listeria monocytogenes Reservoir Environment: soil, water, drains, food production areas. Consumption of heavily contaminated ready-to-eat food products, usually with a high degree of processing, of neutral pH, and with an extended refrigerated shelf life. Gastrointestinal tract of animals and birds. Transmission. Foods associated with transmission have involved dairy products, meat-based products, seafood and vegetableIsolation Hospitalised pregnant women or neonates based products. of positive women should be isolated – inform ICT. Other relevant features Contacts / exposed Clinical surveillance only Exclusion Most cases present as septicaemia and/or meningitis in the immunocompromised, or as an infection of pregnant women and fetus/neonate. Pregnant women present with a series of pyrexial influenza-like illnesses. Some stains of L.monocytogenes also cause a diarrhoeal illness plus fever. Not required Malaria Control of human source Statutorily notifiable. Cause A parasitic disease caused by Plasmodium species. Cases Isolation not required. Reservoir Humans Contacts / exposed There are no public health actions, as the disease does not spread here. However, a comprehensive questionnaire is usually sent to the patients who have been notified Transmission. Anopheles Mosquito bites Other relevant features Imported disease only (at present) Isolation / exclusion Not required Page 43 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Marine biotoxins – marine algal shellfish poisoning syndromes and ciguatera poisoning Control of human source Statutorily notifiable as food poisoning. Person-to-person spread does not occur. Cases Enteric precautions only. Cause Ingestion of toxic algae and retention of the toxins by filter-feeding bivalves and some fish. Concentration of toxins up the food chain by some carnivorous gastropods, crustaceans, and fish. Contacts Clinical surveillance only. Reservoir Toxic seafood. Exclusions None required. Transmission Consumption of toxic seafood. Microbiological clearance None required. Other relevant features The main syndromes are amnesic shellfish poisoning, diarrhetic shellfish poisoning, neurotoxic shellfish poisoning (NSP), paralytic shellfish poisoning (PSP) and ciguatera poisoning. A wide range of gastrointestinal and neurological symptoms is often manifest, for example, respiratory paralysis in PSP, a reversal of hot-cold temperature sensation in NSP and ciguatera poisoning. Symptoms are dose related and mild cases of all these syndromes may only suffer gastrointestinal effects. All the toxins are heat stable and not removed by cooking and processing. There are no known antidotes. Page 44 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Marine biotoxins – scombrotoxic poisoning Control of human source Statutorily notifiable as food poisoning. Person-to-person spread does not occur. Cases None required. Contacts Clinical surveillance only. Exclusions None required. Microbiological clearance None required. Cause Conversion of histidine to histamine by growth of some bacteria. Histidine is naturally present at high levels in the flesh of some fish such as tuna, mackerel and sardines. Reservoir Natural flora of fish and environmental contamination. Transmission Inadequate temperature control of fish at any stage after catching. Histamine is heat stable and not reduced by cooking or processing. Other relevant features The symptoms of some patients are sufficiently severe that they visit accident and emergency departments. Measles Control of human source Statutorily notifiable. Cause Virus (Morbillivirus) Cases Measles is most infectious from 4 days before the appearance of the rash until 4 days afterwards Reservoir Humans Transmission. Respiratory droplets, (airborne) Contacts / exposed For measles, vaccination of susceptible siblings and other close contacts is effective up to 72 hours after contact. HIG may be considered for those babies between 6-8 months or pregnant women with no infection or immunisation history. Other relevant features SSPE (subacute sclerosing pan-encephalomyelitis) is the most severe complication of measles. It is rare occurring in less than 1 in 100,000 cases of measles. Isolation / exclusion School children and others 4 days from onset of rash Page 45 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Meningitis (including septicaemia) Control of human source Statutorily notifiable. This disease is notifiable whether caused by Meningococci or other bacteria (Pneumococci, Listeria) or viruses. Meningococcal septicaemia is notified separately from meningococcal meningitis. Cases Isolated in hospital until treated. Cause Meningococcal bacteria (Neisseria meningitidis) and other bacteria, viruses, and other organisms, Reservoir Humans Transmission. Person to person droplet spread Other relevant features A major cause of public health concern. Contacts / exposed Prophylaxis antibiotics are administered following advice from HPU team. Immunisation may be required depending on type. Isolation / exclusion Siblings and other contacts do NOT need to be excluded from normal activities including school. Mumps Control of human source Statutorily notifiable. Cause Mumps virus (Paramyxovirus) Cases Suggest avoid close contact with susceptible persons. Salivary swabs plus letter should be sent to the GP who should return swab taken to CfI. Immune status of patient should be ascertained. Reservoir Humans Contacts / exposed Immunise contacts with MMR. Note that vaccinations must not be given in pregnancy. Swabs are needed for enhanced surveillance, as many notifications on clinical grounds are erroneous. Transmission. Airborne, droplet, direct contact with saliva. Only moderately transmissible. Other relevant features Preventable through MMR immunisation. Mumps meningitis and encephalitis although usually mild are common features. Isolation / exclusion School children 5 days from onset of swelling Page 46 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Non-cholera vibrios Control of human source Statutorily notifiable as food poisoning. Cases None required. Contacts Clinical surveillance. Exclusions 48 hours after first normal stool. Microbiological clearance None required. Cause Halophilic vibrios (Vibrio parahaemolyticus, V. vulnificus, V. fluvialis, V. hollisae); nonhalophilic vibrios (non-O1 and non-O139 Vibrio cholerae, V. mimicus). V. vulnificus is the most virulent of the non-cholera vibrios and is primarily associated with severe, soft tissue infections or septicaemia, or both, rather than diarrhoea. Reservoir These organisms are part of normal marine flora and proliferate during the summer months. Transmission Transmission of halophilic vibrios is foodborne via consumption of raw or undercooked contaminated shellfish Transmission of nonhalophilic vibrios is via consumption of very large inocula from contaminated water and, occasionally, food. Person-to-person transmission has not been demonstrated. Secondary spread is rare, even where sanitation is sub-optimal, suggesting that the infective dose for normal, healthy individuals is relatively high. Other relevant features Non-cholera Vibrio infections in the UK tend to be imported in travellers returning from warmer climates. Non-O1 V. cholerae does not seem to cause sweeping epidemics, unlike V.cholerae O1 and V. cholerae O139, but explosive outbreaks have been caused by a few non-O1 strains. Genetic differences mediate these differences in epidemiological behaviour. Page 47 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Noroviruses (Norwalk-like viruses [NLV], small round structured viruses [SRSV]) Cause Norovirus genogroup 1 or 2. Control of human source Statutorily notifiable if thought to be food poisoning. Reservoir Human gastrointestinal tract. Cases Enteric precautions with particular attention to environmental contamination, relating to vomit, especially of toilets and wash hand basins, as well as soft furnishings. Cases occurring in institutions should be isolated where practicable. Contacts Clinical surveillance. Do not transfer patients during incubation period. Authorities must satisfy themselves of the adequacy of hygiene and toilet facilities and arrangements. Exclusions 48 hours after first normal stool. Transmission Person-to-person by the faecal-oral route; risk of infection from aerosols or environmental contamination due to projectile vomiting. Water and foods contaminated by a case/excreter. Particular problems arise with ready-to-eat foods, which are extensively handled, by a case or excreter during preparation (e.g. salads & sandwiches). Other relevant features Infectivity lasts for 48 hours after resolution of symptoms. Person-to-person spread is very common and may be difficult to contain. Soft furnishings may need to be steam cleaned. Soiling with vomit should ideally be cleaned to a radius of two metres and people taken out of the area until it is done. Microbiological clearance None required. Ophthalmia Neonatorum Control of human source Not statutorily notifiable according to the new regulations. Cases Prevent contact with other neonates until treated. Contacts / exposed Clinical surveillance Cause Neisseria gonorrhoea (an Sexually Transmitted) or Chlamydia trachomatis or other bacteria such as staphylococci Reservoir - Human Transmission. Direct contact with infected birth passage. Secondary spread by direct contact with eye discharge. Other relevant features Acute infection of the eye in first three weeks of life. Gonorrhoea is now uncommon in pregnant women but genital Chlamydia may be present in up to 5% of women. Page 48 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Plague Control of human source Statutorily notifiable. Maybe considered a potential for CBRN. Cause Yersinia Pestis Cases Isolation required Reservoir Pathogen of Rodents Contacts / exposed Transmission. By the bite of fleas infected with the organism. Antibiotic prophylaxis for household and close contacts. Treat patient, clothing and possessions with appropriate insecticides. In the cae of urban rats, houses, outhouses and household furnishings should also be disinfected. Identify likely source of infection; if abroad consider treating traveling companions with insecticide. if in the UK (unlikely) treat entire house, furnishings and inhabitants with insecticide and then act to reduce rat population. Other relevant features Does not occur in the UK, but may be imported from plague – infected areas in Africa, Asia and South America, usually at times of epidemics. It is one of the diseases covered by International Regulations. Page 49 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Rabies Control of human source Statutorily notifiable. Cause Rhabdovirus. Cases Person to person not documented but theoretically possible. Therefore isolate. Reservoir Wild and domestic animals Transmission. Bites, scratches and licks (present in saliva). Contacts / exposed Consider anti-rabies treatment in contacts that have had an open wound or mucous membrane exposed to patient’s saliva. Other relevant features Several rabies-free countries including UK have rabies-like virus in their bat populations. This includes Australian Bat Lyssavirus in Australia, and European Bat Lyssavirus 1 and 2 in Europe and in the UK. The risk from these viruses is likely to be low because the incidence of acute infection and excretion of virus is rare in bats and because humans are rarely exposed to the most affected bat species (in the UK this is the Daubenton’s bat). Specialist advice should be sought. Relapsing fever Control of human source Not statutorily notifiable under the new regulations. Cause Borrelia recurrententis – bacterium Cases Not after delousing. Reservoir Humans, rodents Contacts / exposed Delousing of clothes Transmission. Tickborne. Not person to person. Isolation / exclusion None required after delousing. Other relevant features Imported. Page 50 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Rubella Control of human source Statutorily notifiable. Cause Rubella virus (Togavirus) Cases Attempt to isolate from pregnant women. Reservoir Humans Contacts / exposed Non-immune pregnant women should consult GP/Obstetrician immediately. Isolation / exclusion Schoolchildren 6 days from onset of rash Transmission. Person to person via respiratory droplets. Other relevant features A cause of severe congenital abnormality. Preventable with MMR vaccine. Rotavirus Control of human source Cases Enteric precautions. Contacts Clinical surveillance only. Authorities must satisfy themselves of the adequacy of hygiene and toilet facilities and arrangements. Cause Rotavirus groups A, B, and C (mainly group A in the UK). Reservoir Human gastrointestinal tract. Exclusions 48 hours after first normal stool. Transmission Person-to-person by faecal-oral route and by environmental contamination. Children and the elderly are at particular risk. Microbiological clearance None required. Other relevant features Person-to-person spread is very common. Page 51 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Salmonella (excluding typhoid and paratyphoid) Control of human source Statutorily notifiable as food poisoning if thought to be foodborne or waterborne. Cases Enteric precautions. Contacts Clinical surveillance. Exclusions 48 hours after first normal stool. Microbiological clearance None required. Cause Salmonella spp. There are some 2,500 serotypes of Salmonella, of which the two most commonly identified in the UK, the rest of Europe and the United States, are Salmonella enterica serovar Enteritidis and S. Typhimurium. Reservoir Salmonellosis is a zoonosis. Salmonella spp. is found in the gastrointestinal tract of a wide variety of wild and domestic animals, birds, reptiles and amphibians. In humans chronic carriage is rare, but organisms are excreted by convalescent carriers, mild and unrecognised cases. Children aged less than 5 years may shed organisms for up to a year (median 10 weeks). Over the age of 5 years the maximum duration of shedding appears to be up to 12 weeks (median 4 weeks). Transmission Transmission is predominantly via contaminated food, where very many food vehicles have been implicated, although waterborne outbreaks have been documented. Exotic Salmonella infections are documented following exposure to exotic pets, especially reptiles (up to 90% of reptiles are Salmonella carriers). Person-to-person transmission is important, especially where cases have diarrhoea. Infants and faecally incontinent adults pose a greater risk of transmission than do asymptomatic carriers. Hospital transmission from patients to staff has been associated with handling of soiled linen, failing to comply with barrier nursing and faecally incontinent patients. On the other hand hospital transmission from healthcare workers to patients appears to be low if infection control measures are strictly adhered to. The risk of transmission to neonates and infants from family members, who are chronic carriers or who have been recently infected, is high. Infected (but not necessarily symptomatic) food handlers have been implicated in outbreaks of salmonellosis. Other relevant features Although volunteer studies suggest that the infective dose may be fairly high, data from outbreaks suggest that low doses (<103) may produce illness and that the ingested dose is an important determinant of incubation period, symptoms, and disease severity. Page 52 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Scarlet fever Control of human source Statutorily notifiable Cause Streptococci (beta-haemolytic group A). Cases Strict isolation not required after starting treatment Reservoir Man Contacts Clinical surveillance. Consider prophylactic antibiotics in closed communities. Swabbing may be indicated. Transmission Direct contact and droplet spread Other relevant features Associated with long term heart valve damage. Exclusion Recommend 24 hour exclusion from school if appropriate treatment has been initiated. Shigellosis (see additional policy) Control of human source Clinical dysentery is notifiable. Personto-person spread is common. Cases Enteric precautions. Contacts Contacts in risk groups A to D of cases of S. dysenteriae, S. flexneri, S. boydii should be screened microbiologically. Otherwise clinical surveillance only. Authorities must satisfy themselves of the adequacy of hygiene and toilet facility arrangements. Hand washing by children must be supervised in nurseries and infant schools. Cause Organisms of the genus Shigella that comprises four species: S. sonnei, S. boydii, S. dysenteriae and S. flexneri. Reservoir Human gastrointestinal tract. Transmission Faecal-oral from cases with diarrhoea, particularly in households, children’s nurseries and infant schools. Occasionally spread by food and water, contaminated by cases. Other relevant features S. sonnei is endemic in the UK and usually only causes a mild illness. S. boydii, S. dysenteriae and most S. flexneri infections, originate outside the UK and frequently present clinically as Exclusions S. sonnei: 48 hours after first normal stool. dysentery (diarrhoea with blood, mucous and S. dysenteriae, S. flexneri, S. boydii pus). S. dysenteriae 1 may be associated with (microbiological clearance). serious disease including toxic mega colon and the haemolytic uraemic syndrome. Microbiological clearance Cases and contacts of S. dysenteriae, S. flexneri, S. boydii in risk groups A to D – two negative faecal specimens taken at intervals of not less than 48 hours. Page 53 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Smallpox Control of human source Statutorily notifiable. Considered potential problem for CBRN – public health emergency Cases Strict isolation Contacts / exposed Isolation. Vaccine available. Cause Variola virus Reservoir Laboratories only Transmission. Person to person Other relevant features Global eradication certified 1979. Isolation / exclusion Isolation and discussion with outbreak control / major incident team. Staphylococcus aureus food poisoning Control of human source Statutorily notifiable as food poisoning. Person-to-person spread does not occur. Cases Enteric precautions. Contacts Clinical surveillance. Exclusions 48 hours after first normal stool. Risk group C – exclude food handlers with septic lesions on exposed skin from work until successfully treated. Nasal carriers do not usually need to be excluded. Microbiological clearance None required after lesions are healed. Cause: Staphylococcus aureus enterotoxins. Reservoir: Infected exposed skin lesions, nostrils, or fingers of food handlers. Rarely, infected animals. Transmission From skin flora or infections in food handlers of cooked foods such as ham, meat, poultry, fish, prawns, and cream cakes which are then stored at room temperature for more than two hours and eaten cold. Some outbreaks are associated with canned foods contaminated after processing. Since the toxins are heat stable, outbreaks have been associated with heat-treated or dried foods. Other relevant factors Some 25% people may carry S. aureus in their nose. Page 54 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Tetanus Control of human source Statutorily notifiable. Cause Toxin produced by Clostridium tetani bacterium. Cases No isolation Reservoir Intestines of horses and other animals Contacts / exposed No precautions required, but remind about immunisation. Transmission. Tetanus spores enter through puncture wounds – particularly road injuries. Isolation / exclusion None required. Other relevant features Preventable by immunisation. Tuberculosis Control of human source Statutorily notifiable Cause Mycobacterium tuberculosis Cases Isolation during early treatment for those with smear positive TB. Otherwise most treated at home. Reservoir Humans primarily (other animal types) Transmission Inhalation of droplets. Contacts/exposed Contact tracing and treatment. This process managed by the respiratory department. Isolation/Exclusion Consideration of health care workers, and those working in schools etc. Risk assessment conducted by Respiratory teams. Page 55 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Typhus fever Control of human source Statutorily notifiable. Cause Rickettsia prowazekii (obligate parasite bacterium) Cases Isolation not required. Reservoir Humans Contacts / exposed None required. Clinical surveillance for similarly exposed. Insecticides in endemic areas. Transmission. Body louse. Not person to person. Other relevant features A disease of tropical highlands Isolation / exclusion None required. Viral Haemorrhagic fever Control of human source Statutorily notifiable. A public health emergency Cause A variety of viruses (including Lassa, Ebola, Marburg, Crimean-Congo) Cases Isolation in special isolation unit Reservoir Animal reservoirs Contacts / exposed Clinical surveillance Transmission. Bites, contact, person to person via blood and body fluids. Isolation / exclusion Asymptomatic contacts isolated at home. Other relevant features Imported diseases requiring rapid communication between departments. Page 56 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Whooping cough (pertussis) Control of human source Statutorily notifiable. Cause Bordatella pertussis Cases Suspected cases should be removed from the presence of young children and infants, especially non-immunised infants, until the patients have received at least 5 days of a minimum 14 day course of antibiotics Reservoir Humans Contacts / exposed Non-immunised contacts may require antibiotics and immunisation. Their household contacts may also require antibiotics. Transmission. Direct contact and respiratory secretions by airborne route. Other relevant features Parapertussis, caused by the bacterium B. parapertussis, is similar to, although usually milder than pertussis Isolation / exclusion Exclude from nursery or school for five days from starting treatment. Worms (helminths) Control of human source Cases Enteric precautions. Early treatment. Cause, Reservoir, Transmission Worms are classified into three groups: Nematodes (roundworms), cestodes (tapeworms), and trematodes (flukes). Threadworm: keep fingernails short and hands well washed. Regular change of underwear, nightclothes and bedclothes. 1. Nematodes (roundworms) i) Threadworm (Enterobius vermicularis) Eggs present in faeces and on perianal region. Transmission – faecal-oral, on hands and under nails as a result of scratching the itchy perianal region. Clothing, particularly nightwear, and Contacts Threadworm: Treat all household bedclothes may be contaminated. contacts – screening not necessary. ii) Whipworm (Trichuris trichiura) Heavy infection may present as a diarrhoeal illness. Strongyloides: Healthcare workers Eggs must mature for 3 to 5 weeks in soil outside should take particular care with the the host before becoming infective. stools, urine, and other body fluids of iii) Roundworm (Ascaris lumbricoides) patients with hyperinfestation as larvae Eggs must mature in the environment for 1 to 2 are infective via skin penetration. weeks before they become infective. They may remain alive outside the human host from months Taenia solium: Screen serologically to years. household contacts of pork tapeworm for evidence of cysticercosis. iv) Hookworm (Necator americanus, Continued……….. Ancylostoma duodenale) Page 57 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Exclusions Threadworm: exclude cases in risk groups A to D until treated. Taenia solium: Exclude cases in risk groups A to D until two negative stools commencing 1 and 2 weeks post treatment. Microbiological clearance Taenia solium: two negative stools at 1 and 2 weeks post treatment for cases in groups A to D. Infection of humans is via skin penetration by larvae, as the eggs passed in faeces have to mature in soil for seven to eight days to form infective larvae. v) Strongyloides stercoralis Larvae passed in the stool are usually not infective until they mature. However, patients with severe immunodeficiency and the Strongyloides hyperinfestation syndrome pass infective larvae in stool and sometimes urine, which are infective to humans by skin contact. 2. Cestodes (tapeworms) i) Eggs of the fish tapeworm Diphyllobothrium latum and the beef tapeworm Taenia saginata are not infective to humans as the lifecycle involves an intermediate host. ii) Eggs of Taenia solium, the pork tapeworm can produce cysticercosis in humans, a potentially dangerous condition. NB The eggs of Taenia solium and Taenia saginata are morphologically identical. Speciation can be made only by examining segments of the worm itself. Taenia saginata is seen much more commonly than Taenia solium in the UK. iii) Eggs of the dwarf tapeworm Hymenolepis nana are infective to humans. 3. Trematodes (flukes) i) Trematode eggs are not directly infective to humans as the lifecycle involves one or more intermediate hosts. Other relevant features Apart from the threadworm Enterobius vermicularis, intestinal helminth infections in the UK are usually seen as imported cases Page 58 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Yellow fever Control of human source Statutorily notifiable. Cause Flavivirus Cases Universal precautions for blood and body fluids. Reservoir Humans, monkeys. Contacts / exposed No action required unless exposure to the vector (not in this country). Transmission. Mosquito bites (Aedes aegypti) Other relevant features Imported. Usually a differential diagnosis problem for viral haemorrhagic fevers. Yersiniosis Control of human source Statutorily notifiable if thought to be the cause of food poisoning. Cause Yersinia enterocolitica; occasionally Y. pseudotuberculosis. Cases Enteric precautions. Reservoir Gastrointestinal tract of many species of wild and domestic animals and birds, usually asymptomatic. Contacts Clinical surveillance. Exclusion 48 hours after first normal stool. Microbiological clearance None required. Transmission Contaminated food and water (organisms can multiply in food at 4°C). Direct contact with infected animals. Person-to-person spread may occur. Particular association with raw pork and pork products. Other relevant features Many cases in children present as abdominal pain that mimics appendicitis. Not all strains are pathogenic and Y. enterocolitica can be isolated from asymptomatic individuals. Page 59 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Appendix 1b – Toxins Chemical Food borne Illness The list on the next few pages is a selection of foodborne illness caused by chemicals. The list is not exhaustive, but attempts to give an idea of the wide variety of ways in which chemicals in food can be implicated in poisoning episodes. Examples of food being contaminated by chemicals are numerous. They may result from: The addition of too much of a chemical allowable in foods e.g. Sorbitol Accumulation of a naturally occurring chemical e.g. Paralytic/diarrhetic shellfish poisoning; Accumulation of inorganic chemicals e.g. Heavy metal contamination of shellfish; The addition of a non-allowable chemical e.g. Toxic oil syndrome; Changes in manufacturing processes allowing a new chemical entity to be copurified with the final product e.g. Chemical contamination of tryptophan producing the Eosinophilic-Myalgic syndrome; Inadequate preparation of foodstuffs e.g. Kidney bean poisoning; The accidental ingestion of toxic species of common foods e.g. Mushrooms; Contamination with herbicides and pesticides which can occur if manufacturers’ instructions are not followed e.g. Anticholinesterase in insecticides; and Malicious contamination. Other routes of contamination can arise as a result of air borne release of chemicals. For instance, following an explosion or a transport accident involving a crashed tanker, direct chemical contamination of foods can take place e.g. growing vegetables and stored grain. Investigations should take cognisance of food grown in the surrounding environment. The effects of chemical contamination of food may be acute or chronic. The definition of acute is arbitrary, but generally refers to a latent time of hours before the onset of symptoms. In acute episodes symptoms produces by chemical poisonings can be similar to those produced by bacterial contamination and it can be impossible to distinguish between them without laboratory investigation. Chronic effects generally occur when the exposure is insidious and over a long time period, although it is possible for the effects of single exposure to a chemical to become manifest at a later date. Because of the insidious nature of chronic exposure it is inevitable that many people will have been exposed before the appearance of symptoms draws attention to the problems. Examples of chronic exposure to chemicals that have produced disastrous effects are methyl mercury contamination of sea food (Minimata Disease) and the toxic oil syndrome in Spain in which illicitly refined rape seed oil was sold as edible oil. Page 60 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 1. CAUSATIVE AGENT(S) Gonyaulax spp., producing saxitoxin Illness Paralytic shellfish poisoning (PSP) Comments Saxitoxins are produced by certain marine algae such as Gonyaulax and Alexandrium species. In summer months Alexandrium species, which are dinoflagellate constituents of plankton, may multiply in seawater with visible effects (eg. Red tides). Filter feeding bivalve shellfish and some crustacea consume these algae and can concentrate the saxitoxins to hazardous levels. Sources Mussels, clams, oysters, scallops and crabs Symptoms Neurological symptoms – dizziness, tingling, drowsiness, headache, muscular paralysis, even respiratory paralysis and death – within a few hours of ingestion. Occasional gastrointestinal symptoms. Duration of illness Dose dependent Incubation period/onset time 30 mins – 2 hours (dose dependant) Diagnosis PSP analysis by specialised laboratories. Action level is 80μg/100g of shellfish Control Closure of shellfish beds Page 61 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 2. CAUSATIVE AGENTS(S) Prorocentrum and Dinophysis spp., producing okadaic acid Illness Diarrhetic shellfish poisoning (DSP) Comments Sources Associated with red tides; however not all algal blooms are red and their proliferation can be missed as a consequence. These small dinoflagellate plankton are consumed by shellfish which concentrate the constituent toxic chemical known as okadaic acid. Additional toxins designated DTX1,2,3, pectenotoxins 1-6 and yessotoxin. Consumption of contaminated bivalve shellfish and crustacea Symptoms Diarrhoea, nausea, vomiting and abdominal pain Duration of illness 3-4 days Incubation period/onset time 30 minutes to not more than 12 hours Diagnosis Detection of DSP toxin in shellfish Control Closure of shellfish beds Page 62 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 3. CAUSATIVE AGENT(S) Diatoms (phytoplankton) producing domoic acid. Illness Amnesic shellfish poisoning (ASP) Comments Filter feeding shellfish live off the small particles of organic matter that they filter from the seawater, including small plants or animals. Some of the small plants (phytoplankton) contain toxins. When the mollusc digests the toxic organism, the toxin is retained largely in the digestive gland. The toxin is water soluble and relatively rapidly cleared from most shellfish but not others such as the Atlantic scallop (Placopecten magellanicus), which appears to accumulate the toxin over long periods of time. The toxin is heat-stable and not destroyed by cooking. Sources Mussels, clams, scallops and anchovies Symptoms: Nausea, vomiting, abdominal cramps and diarrhoea; headache. If severe, temporary or permanent brain damage may occur. The syndrome is complex but the most notable simple characteristic is a loss of shortterm memory, hence the toxin is sometimes called Amnesic Shellfish Poison (ASP). Deaths may occur. Duration of illness Few days or longer in severe cases Incubation period/onset time 30 mins – 6 hours (dose dependant) Diagnosis Control Domoic acid analysis by specialised laboratories (HPLC or mouse bioassay). Action level is 40μg/100g of edible parts of shellfish Closure of shellfish beds unless levels of toxin below 20 µg/g of edible parts of shellfish Page 63 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 4. CAUSATIVE AGENT(S) Bacterial spoilage of scombroid (mackerel, tuna) and other fish, producing histamine and related chemicals Illness Scombrotoxin fish poisoning Comments Sources Spread Symptoms Typically associated with consumption of fish, which may contain concentrations of histamine and histamine-like substances, but has also been caused by other foods (eg Swiss cheese). Spoilage bacteria which multiply in unrefrigerated food Spoiled fish that has been canned can pose a threat as the toxin can resist canning temperatures. Contaminated fish are not usually organoleptically detectable. Diarrhoea, flushing, headache, sweating sometimes accompanied by nausea, abdominal pain, burning in the mouth. Duration of illness 6-24 hours Incubation period/onset time 10 mins – 2 hours (approximately) Diagnosis Control Detection of excess levels of histamine in fish. Recently it has been suggested that histamine is not the best marker. However, other markers are not yet in general use. All open scombroid fish products should be kept chilled, and canned fish should be pre-chilled to a maximum of 5C before handling, to reduce the likelihood of histidine-histamine conversion by bacteria introduced during handling. All cans (especially large catering cans) should be pre-chilled before opening and handling product in preparation of salads, mayonnaise mixes, etc. All made-up or mixed scombroid fish products should be kept chilled throughout their storage life until usage or disposal. Page 64 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 5. CAUSATIVE AGENT(S) Heavy metals, for example: antimony, zinc, lead, copper Illness Heavy metal poisoning Sources Symptoms Consumption of contaminated food. Antimony – acid foods stored in enamel Zinc – acid foods stored in galvanised containers Lead – food stored in lead, or contaminated with lead accidentally Copper – high acid foods stored in copper utensils The association of gastrointestinal symptoms (bloody diarrhoea, abdominal pain, nausea, vomiting and upper gastrointestinal haemorrhage) with metallic taste in the mouth. Page 65 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 6. CAUSATIVE AGENT(S) Toxins from natural foods, for example; 1) phytohaemagglutinin, found in red kidney beans 2) Toxins, probably glycoalkaloids (solanine and chaconine) in green or sprouting potatoes. Illness Natural toxin food poisoning Comments Toxigenicity Sources Symptoms Raw red kidney beans must be soaked for at least 12 hours and then cooked thoroughly (boiled for at least 10 minutes). Canned red kidney beans can be eaten without further cooking. Potato glycoalkaloids are not destroyed by cooking or processing. Some doubts have been expressed as to whether solanine is the causative agent. 1) No data available 2) Adverse effects have been associated with the ingestion of potatoes containing total glycoalkaloids in excess of 200mg/kg. 1) Red kidney beans, butter beans and lentils 2) Green or sprouting potatoes 1) Nausea, vomiting, followed by abdominal pain and diarrhoea 2) Unpleasant taste, bitterness, burning sensation in mouth and throat, headache, diarrhoea and vomiting, and feelings of general malaise Duration of illness 3 hours – 6 days Incubation period/onset time 30 mins – 12 hours (approximately) Diagnosis Control 1) Detection of haemagglutinins in food 2) Glycoalkaloid concentrations should not exceed 200mg/kg. Identification of solanine best carried out by specialised analytical laboratories. Proper cooking of all bean varieties Potatoes should be stored in a cool, dark place Page 66 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 7. CAUSATIVE AGENT(S) Amanita phalloides (death cap), Amanita verna and Amanita virosa, some Galerina and Lepiota species Illness Mushroom poisoning Comments These fungi contain a number of cyclopeptides and amatoxins responsible for the poisoning that follows consumption. Toxigenicity Variable Symptoms Colic, nausea, vomiting and diarrhoea. There may be a brief period of apparent recovery that is followed by liver and/or kidney failure. Death occurs in 20 –90% of cases. Duration of illness 1-30 days Incubation period/onset time 6 – 48 Hours Diagnosis Identification of causative agent in food, stomach contents or stools. Detection of amatoxins in blood or biological fluids. Monitoring patient hepatic function. Treatment of cyclopeptide poisoning is complicated and needs to be started rapidly. In all cases of mushroom poisoning the possibility of cyclopeptide poisoning must be excluded. Suspected cases of cyclopeptide poisoning should be immediately referred to the Regional Poisons Centre. Page 67 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 8. CAUSATIVE AGENT(S) Amanita muscaria, Amanita pantherina containing ibotenic acid and muscimol Illness Mushroom poisoning Comments Sources Symptoms No commonly eaten fungi are similar to these species but A pantherina can be confused with A rubescens. Ingestion likely to be accidental in children or as a recreational drug in adults. Fungi. Many other fungi also can cause poisoning eg Inocytes, Cortinarius etc. Initially similar to alcohol intoxication, progressing to muscular twitches cramps and hyperkinesia. Visual disturbances, hallucinations, a feeling of floating and a feeling of superhuman strength ma occur. Children may suffer from confusion, hysteria and seizures. Duration of illness <12 hour (approximately) Incubation period/onset time 30 mins – 3 hours (approximately) Diagnosis Symptomatology, fungi in stomach contents or stools Page 68 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 9. CAUSATIVE AGENT(S) Aspergillus flavus and A parasiticus toxins, aflatoxin Illness Aflatoxicosis Comments Sources Symptoms These fungi are found worldwide and grow on practically any substrate. Widespread in soil and air. Occur in some cereal crops prior or harvest. Heavy rains and faulty methods of storage also allow theses fungi to grow especially in grain, peanuts and other nuts. Vomiting, diarrhoea, fever, abdominal pain, jaundice, coma, convulsions, hepatic and renal failure. Death can occur 3 – 10 days after onset of symptoms. Aflatoxins are also carcinogens. Duration of illness >10 days Incubation period/onset time 8 hours or longer Diagnosis Control Isolation of fungi, microscopy, extraction, separation, chromatography, spectrophotometry Crop management, control of moisture during food storage. Page 69 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 10. CAUSATIVE AGENT(S) Anticholinesterase insecticides, including the organophosphorus and carbamate insecticides Illness Pesticide food poisoning Comments Symptoms Diagnosis Although several groups of pesticides have given rise to acute poisoning in various parts of the world, many of these poisonings are due to suicide or accident, and few are caused by food contamination. Aldicarb was responsible for outbreaks of food-borne illness in the USA, Canada and Ireland. Accumulation of the neurotransmitter acetylcholine, causing symptoms such as bradycardia, blurred vision, dyspnoea and muscle fasciculation. In severe cases convulsions may occur. In mild oral poisoning the most prominent symptom is stomach pain, which is often described as cramp-like. Specific antidotal treatment is available: atropine is effective in both organophosphorus and carbamate poisoning; in addition in organophosphorus poisoning, pralidoxime mesylate should be administered. Diagnosis is based upon the characteristic clinical signs and measurement of plasma and erythrocyte cholinesterase levels. Page 70 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Appendix 1c – Factsheets, links and policies Health Protection Legislation (England) Guidance 2010 http://www.dh.gov.uk/prod_consum_dh/gro ups/dh_digitalassets/@dh/@en/@ps/docum ents/digitalasset/dh_114589.pdf London Infectious Disease Outbreak Management Plan. (latest version can be found in the HPA website using the search box) http://www.hpa.org.uk/AboutTheHPA/What TheHealthProtectionAgencyDoes/LocalSer vices/London/RegionalPublications/Infectio nControl/londinfdiseaseoutbreakmgmentpla n/ Food and Environmental Questionnaire S:\Reactive Folder_NEW\Gastro - Katie & Simon\Questionnaires and HPA Lab Guidebook for Environmental Health Officers, Consultants in Communicable Disease Control and Primary Care Groups Barts and the London HPA lab.pdf Aeromonas general D&V leaflet.doc Bacillus cereus / subtilis general D&V leaflet.doc Campylobacter Campylobacter London factsheet for public March 2011.doc Clostridium perfringens general D&V leaflet.doc Cryptosporidium o Policy o Questionnaire o Factsheet S:\Reactive Folder\ Cryptosporidium London fact sheet for Questionnaires\Cryptosporidiosis public March 2011.doc case questionnaire r S:\Reactive Folder\ Policies\Cryptosporidium policy NE London_2010.doc Giardia Giardia London factsheet for public March 2011.doc Salmonella non- Typhoid or Paratyphoid Salmonella London factsheet for public march 2011.doc Page 71 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Shigella non-sonnei Shigella London factsheet for public March 2011.doc Taenia solium (worms) (link to NHS information) http://www.nhs.uk/conditions/Tapeworm -infections/Pages/Introduction.aspx Vibrio non-cholera Viral gastroenteritis Yersiniosis general D&V leaflet.doc Norovirus.doc Rotavirus Factsheet_TM.doc general D&V leaflet.doc Hepatitis A o Policy o Questionnaire o Factsheet Hepatitis A policy July 2010.doc S:\Reactive Folder\ Questionnaires\Hepatitis A London 2011 V3 0. S:\Reactive Folder\ Factsheets\Hepatitis A.doc Legionella o Policy o Questionnaire o Factsheet Legionnaires' Disease S:\Reactive Folder\ Policy_2010.doc Questionnaires\Legionella Questionnaire(elect S:\Reactive Folder\ Factsheets\Legionnaires.doc Listeria Monocytogenes o Policy o Questionnaire o Factsheet Listeria 2010.doc S:\Reactive Folder\ Questionnaires\Listeria trawling questionnaire S:\Reactive Folder\ Factsheets\Listeria.doc Salmonella Typhoid and Paratyphoid o Questionnaire o Operational guidance o Factsheet o Warn and inform letter S:\Reactive Folder\ S:\Reactive Folder\ Policies\Typhoid and paratyphoid Policies\Typhoid policyand andparatyphoid supportive documents policy and su J S:\Reactive Folder\ S:\Reactive Folder\ Policies\Typhoid and paratyphoid Policies\Typhoid policyand andparatyphoid supportive documents policy and su J Page 72 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Shigella non Sonnei o Policy o Factsheet S:\Reactive S:\Reactive Folder\ Folder_NEW\Shigella -Factsheets\Shigella Vicky\Shigella PolicyLondon Marchfactsheet 2012.doc for publ S:\Reactive Folder_NEW\Shigella - Vicky\March 2012_Enhanced Shig VTEC O157 o o o o Operational manual Supporting evidence Questionnaire Factsheet VTEC opertaional VTEC Support manual Feb 2011.pdf Document - Background evidence for PH Mana S:\Reactive Folder\ S:\Reactive Folder\ Questionnaires\VTEC Questionnaire Factsheets\E.Coli - electronic 0157 fact version sheet-Nov08.doc 2011.doc Page 73 of 74 North East and North Central London HPU JOINT INFECTIOUS DISEASE PROTOCOL Revised September 2011 Page 74 of 74