The prevalence of trypanosomes in tsetse flies in Handeni district
advertisement

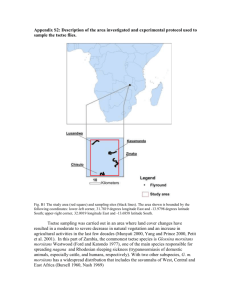
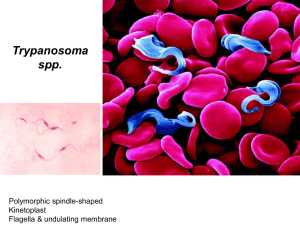
The prevalence of trypanosomes in tsetse flies in Handeni district and Ngorogoro Conservation Area. Name: Maike van der Geest Supervisor: F.J.C.M. van Eerdenburg Supervisors Tanzania: Z. Lyimo and F. Mramba Date: March 2011 The prevalence of trypanosomes in tsetse flies in Handeni district and Ngorogoro Conservation Area Index 1. Abstract 2. Introduction 3. Materials en methods 3.1. Field site 3.2. Field collection 3.3. Fly dissection 4. Results 4.1. Results of both Handeni district and Ngorogoro Conservation Area 4.2. Results of Handeni district 4.3. Results of Ngorogoro Conservation Area 4.4. A comparison between Handeni district and Ngorogoro Conservation Area 5. Discussion 2 The prevalence of trypanosomes in tsetse flies in Handeni district and Ngorogoro Conservation Area 1. Abstract Tsetse flies (Glossina spp.) are a big problem in sub-Saharan Africa because they are the most common vector for trypanosomes. In this part of Africa they threat human health and limit rural development by transmitting the trypanosome parasites that cause the fatal diseases of Nagana (or AAT; African Animal Trypanosomiasis) in domestic animals and Sleeping Sickness (or HAT; Human African Trypanosomiasis) in human. Direct losses from trypanosomiasis in cattle include mortality, morbidity, decreased milk and meat production, impaired fertility and the costs of tsetse and trypanosomiasis control operations. Indirect losses include the farmers’ responses to the risk of the disease. A study on the prevalence of trypanosomes in tsetse flies was conducted from September 2010 to November 2010 in Handeni district and Ngorogoro Conservation Area, Tanzania. The fly-survey was conducted by using biconical, pyramidal and NGU traps and revealed that two tsetse species namely Glossina pallidipes and G. swynnertoni were found. The prevalence of trypanosomes in tsetse flies and the apparent density of these flies were determined. A total of 375 flies were dissected, and revealed the presence of trypanosomes in tsetse flies in these areas. The overall prevalence of trypanosomes in tsetse flies was 3.5%. The prevalence in Handeni district (4.49%) was higher then the prevalence in Ngorogoro Conservation Area (2.73%). Overall the results suggested that the tsetse density is low and the prevalence of trypanosomes in these tsetse flies is low in this time of year. This needs to be considered when developing area- specific strategies for future management of tsetse- transmitted animal trypanosomiasis. 3 The prevalence of trypanosomes in tsetse flies in Handeni district and Ngorogoro Conservation Area 2. Introduction Tsetse flies (Glossina spp.) are a big problem in sub-Saharan Africa because they are the most common vector for trypanosomes. In this part of Africa they threat human health and limit rural development by transmitting the trypanosome parasites that cause the fatal diseases of Nagana (or AAT; African Animal Trypanosomiasis) in domestic animals and Sleeping Sickness (or HAT; Human African Trypanosomiasis) in human. Tsetse fly These diseases are often fatal without an adequate drug treatment. [10] In sub-Saharan Africa at least 46 million cattle are exposed to the risk of tsetse-borne trypanosomes. The disease is costing sub-Saharan African producers and governments at least $35 million per year only for treatments and the total costs contains about $192 to $960 million. Direct losses from trypanosomiasis in cattle include mortality, morbidity, decreased milk and meat production, impaired fertility and the costs of tsetse and trypanosomiasis control operations. Indirect losses include the farmers’ responses to the risk of the disease. This includes the reduction and the exclusion of livestock from tsetse-infested grazing lands and reduced crop production due to insufficient animal draft power.[11] In Tanzania, bovine tsetse-borne trypanosomiasis is one of the two most important diseases that cause enormous losses in livestock productivity. [9] The Nagana disease is a name used to describe all the tsetse fly transmitted trypanosome infections in domestic animals in sub-Saharan Africa. The subgenera Duttonelle, Nannomonas and most important Trypanozoon contain the species that causes most cases of the disease of Nagana in livestock. The subgenus causing sleeping sickness in human is only Trypanozoon. [6]This are the subspecies of Trypanosoma brucei rhodesiense and T.b. gambiense. The major pathogenic trypanosome species in animals are T. congolense in cattle and camels, T. vivax in cattle and T. brucei brucei in horses, camels and dogs. T. brucei brucei also cause some less severe disease in cattle, goat and sheep. [8] T. vivax [2] T. congolense [18] T. brucei brucei [3] Each of the trypanosome species causing the disease of Nagana in livestock is capable of a cycle of development in the tsetse fly and because of this it is possible to find infective trypanosome species in the mouthparts of the tsetse fly. This is called the cyclical method of development. The mechanical method of transmission is when the trypanosomes taken up with the blood survives for a short period of time in the mouth parts of the fly and infects another animal when the fly feeds again. So the trypanosomes do not undergo any development in the vector with the mechanical method of transmission. [6] Tsetse flies are bloodsucking dipteran flies (Glossinae) that act as vectors of the several species of trypanosomes. The trypanosome hides in the fly’s saliva and are transmitted when the fly bites the host. [12] Both males and females act as vectors for tryanosomiasis. [13] In the following figure the life cycle of the trypanosomes can be seen. This is a figure for human Sleeping Sickness, but it describes the life cycle of the trypanosomes in all mammalian hosts. 4 The prevalence of trypanosomes in tsetse flies in Handeni district and Ngorogoro Conservation Area During a blood meal on the mammalian host, an infected tsetse fly (genus Glossina) injects metacyclic trypomastigotes into skin tissue. The parasites enter the lymphatic system and pass into the bloodstream . Inside the host, they transform into bloodstream trypomastigotes , are carried to other sites throughout the body, reach other blood fluids (e.g., lymph, spinal fluid), and continue the replication by binary fission . The entire life cycle of African Trypanosomes is represented by extracellular stages. The tsetse fly becomes infected with bloodstream trypomastigotes when taking a blood meal on an infected mamalian host ( , ). In the fly’s midgut, the parasites transform into procyclic trypomastigotes, multiply by binary fission , leave the midgut, and transform into epimastigotes . The epimastigotes reach the fly’s salivary glands and continue multiplication by binary fission . The cycle in the fly takes approximately 3 weeks. [5] Tsetse flies and especially Glossina morsitans (G. morsitans) and G. pallidipes feed on game animals like warthog, bush pig, kudu, bushbuck, elephant, rhinoceros and the African buffalo. These animals act as a reservoir of infection, because they are often subclinically infected. [16] In Tanzania there 33 different species of tsetse flies. Not all species are capable of transmitting trypanosomias. Species who are capable of transmitting the trypanosomes are; G. morsitans, G. pallidipes, G. swynnertoni and G. austeni occupying the savannah areas; G. fuscipes, G. palpalis and G. Tachinoïdes occupying riverine and lake areas and G. brevipalpis and G. longipennis occupying forest areas. [9] 5 The prevalence of trypanosomes in tsetse flies in Handeni district and Ngorogoro Conservation Area [1] The female tsetse fly only mates once in her life. She produces a full grown larva every 9 days, which is not eating outside of the fly. The larva will pupates rapidly in sandy soil and in 30 to 40 days it will hatch. Female flies produce their first offspring 16 days after hatching. [13] Especially the interaction between human, livestock and game animals is very important and likely will be more important in the future. This is because of agricultural development and because the African population is expanding rapidly. This is the reason for habitat disappearance for game animals and tsetse flies. Because of this they have to stick to protected areas in Tanzania like national parks, game reserves and forest reserves. Human and livestock living in these areas and at the edge of these areas have to deal with the tsetse flies and the diseases they cause. [15] The main reason for studying trypanosomiasis in cattle and tsetse flies is the economical one. [14] Livestock, especially cattle, is very important to the population of Tanzania. For most people livestock is their source of food, draught power, money and cattle plays an important role in cultural affairs. [16] Because of this it is important to control trypanosomiasis in cattle. The purpose of this study was to establish the prevalence of trypanosomosis in tsetse flies in Ngorogoro Conservation Area and in Handeni district. 6 The prevalence of trypanosomes in tsetse flies in Handeni district and Ngorogoro Conservation Area 3. Materials and methods 3.1. Field site The field work was organized with the Tsetse and Trypanosomiasis Research Institute (TTRI), Tanga, Tanzania. Tanzania has a bimodal rain pattern with 83% of the rain falling between late February and the end of May and short rains between November and January. Two field sites were used: Handeni, one of the eight districts of Tanga region (latitude 5° S, longitude 38° E and an elevation of 830 meters above sea level) and Ngorogoro Conservation Area in the north region of Tanzania (latitude 3° S, longitude 35° E and an elevation of 1700 meters above sea level). Handeni district is divided into 7 divisions, 19 wards and 112 villages with a population of 248,663 people. The vegetation type is mainly acacia wooded grassland and shrubs. Ngorogoro Conservation Area is an UNESCO World Heritage Site in the crater highlands area in the west of Arusha. This area is famous because it is the only area in Tanzania with a protection status for wildlife and at the same time allowing human to keep cattle in this area. [19] This area is the home for the big five of rhinoceros, lion, leopard, elephant and buffalo and also zebra, gazelle, impala, giraffe, warthog, wildebeest, monkeys and many birds. The vegetation type is mainly acacia wooded grassland and shrubs. There are 26,000 Maasai living in this area with 285,000 head of cattle. Although this is a guess, because it is difficult to count the amount of people and cattle living here. In both areas the farmers had local breeds of cattle, namely Boran or the Tanzania shorthorn Zebu. These animals were sent out for grazing and drinking every day and were not supplemented with concentrates. The use of acaricides against flies and ticks are very common. 3.2. Field collection Mostly pyramidal and biconical and some NGU traps were used to catch the flies and underneath every single trap two types of chemical attractant were placed; a bottle of acetone with a hole in the top lid and a 4 ml sachet of 3-n-propylphenol, octenol and 4-methyl-phenol and at a ratio of 8:4:1. These attractants resembles respectively cow breath and cow urine. The blue color of the trap is to attract the fly and the black color of the trap is to induce the flies to settle.[16] [10] The traps were placed 100-200 meters apart and under the shade of trees to avoid fly mortality caused by the heat of the sun. NGU trap Biconical trap Pyramidal trap The living or dead but fresh enough flies were stored in nets and transported to the laboratory for dissection the same day. Before dissection the flies were sorted according to species and sex which was based on morphological features. The sexes are easily recognized because the male hypopygium is well developed. The species were identified by using the key described in ‘infectious diseases of livestock’. [16] 3.3. Fly dissection Before dissecting the flies the living ones were killed by squeezing the thorax and dissected when they are still fresh. Only non- teneral flies were dissected. After the fly was killed, the wings and legs were removed, and the body placed on a microscope slide. Then proboscis, midgut and salivary gland were dissected from every fly on a slide in an immersion of 0.9% saline to keep the trypanosomes alive. 7 The prevalence of trypanosomes in tsetse flies in Handeni district and Ngorogoro Conservation Area The proboscis could be separated using dissecting needles by applying pressure to the bulb (thecal bulb) at the base of the proboscis. The three parts of the proboscis: the labium, hypopharynx and labrum could then be separated using needles and spread apart Special attention is given to examining the hypopharynx for trypanosomes. [20] To dissect the salivary gland it was necessary to remove the second segment of the abdomen. If the fly was lactating there was some fat in the abdomen and a large transparent structure, the salivary gland. This gland was removed using needles. After removing the salivary gland, it was easy to remove the midgut. After dissection, the proboscis, midgut and salivary gland were examined for the presence of trypanosomes using a Zeiss axiostar light microscope. Because trypanosomes belonging to different subgenera develop in different parts of the fly, one can often recognize the subgenus involved by the location in the fly. T. brucei can be found in proboscis, midgut and salivary gland, T. congolense can be found in proboscis and midgut and T. vivax can only be found in the proboscis. There are also some morphological differences between the different species of trypanosomes. T. brucei has a developed flagella and a small kinetoblast. T. congolense has a poor developed flagella and a large kinetoblast. T. vivax has a well developed flagella. This makes T. vivax the fastest, followed by T. brucei and T. congolense. 8 The prevalence of trypanosomes in tsetse flies in Handeni district and Ngorogoro Conservation Area 4. Results 4.1. Results of both Handeni district and Ngorogoro Conservation Area A total of 375 flies were dissected over the study period. The number of tsetse dissected from Ngorogoro Conservation Area (n= 219) district is higher than the number of flies dissected from Handeni district (n= 156). The species isolates in the survey were G. pallidipes, G. morsitans and G. swynnertoni. Only 2 G. morsitans were dissected. They were both negative for trypanosomes in proboscis, salivary gland and midgut, so they do not influence the results. Therefore it was not necessary to exclude these results for analysis. Species captured in both Ngorogoro Conservation Area and Handeni district Frequency Valid Percent Valid Percent Cumulative Percent G. pallidipes 268 71.5 71.5 71.5 G. morsitans 2 .5 .5 72.0 G. swynnertoni 105 28.0 28.0 100.0 Total 375 100.0 100.0 Prevalence of trypanosomiasis in both Ngorogoro Conservation Area and Handeni district Frequency Valid negative positive Total Percent Valid Percent Cumulative Percent 362 96.5 96.5 96.5 13 3.5 3.5 100.0 375 100.0 100.0 ` Frequency of males and females captured in both Ngorogoro Conservation Area and Handeni district Frequency Valid Percent Valid Percent Cumulative Percent female 252 67.2 67.2 67.2 male 123 32.8 32.8 100.0 Total 375 100.0 100.0 Off all dissected females 4.76% (n=12) were tested positive for trypanosomes. Off all dissected males 0.8 % (n=1) were tested positive for trypanosomes. 9 The prevalence of trypanosomes in tsetse flies in Handeni district and Ngorogoro Conservation Area 4.2. Results of Handeni district Species captured in Handeni district Frequency Valid Percent Valid Percent Cumulative Percent G. pallidipes 154 98.7 98.7 98.7 G. morsitans 2 1.3 1.3 100.0 156 100.0 100.0 Total Prevalence of trypanosomiasis in Handeni district Frequency Valid negative Valid Percent Cumulative Percent 149 95.5 95.5 95.5 7 4.5 4.5 100.0 156 100.0 100.0 positive Total Percent Frequency of males and females captured in Handeni district Frequency Valid Percent Valid Percent Cumulative Percent female 86 55.1 55.1 55.1 male 70 44.9 44.9 100.0 Total 156 100.0 100.0 Off all dissected females 6.98% (n=6) were tested positive for trypanosomes. Off all dissected males 1.42 % (n=1) were tested positive for trypanosomes. Positive flies captured in Handeni district species G. pallidipes site negative proboscis Total G. morsitans Total 147 2 149 7 0 7 154 2 156 All positive flies captured in Handeni district were G. pallidipes. Trypanosoma were only found in proboscis. This means that all Trypanosomes were T. vivax. 10 The prevalence of trypanosomes in tsetse flies in Handeni district and Ngorogoro Conservation Area 4.3. Results of Ngorogoro Conservation Area Species captured in Ngorogoro Conservation Area Frequency Valid Percent Valid Percent Cumulative Percent G pallidipes. 114 52.1 52.1 52.1 G. swynnertoni 105 47.9 47.9 100.0 Total 219 100.0 100.0 In Ngorogoro Conservation Area no G. morsitans were captured. Prevalence of trypanosomiasis in Ngorogoro Conservation Area Frequency Valid negative Valid Percent Cumulative Percent 213 97.3 97.3 97.3 6 2.7 2.7 100.0 219 100.0 100.0 positive Total Percent Frequency of males and females captured in Ngorogoro Conservation Area Frequency Valid female Percent Valid Percent Cumulative Percent 166 75.8 75.8 75.8 male 53 24.2 24.2 100.0 Total 219 100.0 100.0 Off all dissected females 3.61% (n=6) were tested positive for trypanosomes. No males were tested positive for trypanosomes during dissection. Positive flies captured in Ngogogoro Conservation Area species G. pallidipes G. swynnertoni site negative Total 112 101 213 proboscis 1 0 1 midgut 1 4 5 114 105 219 Total The positive flies captured in Ngorogoro Conservation area were G. pallidipes and G. swynnertoni. Trypanosoma were found in proboscis and midgut. This means that the G. pallidipes fly which had only an infection in the proboscis was infected with T. vivax. The other flies which were tested positive on the midgut where infected by T. brucei or T. congolense. 11 The prevalence of trypanosomes in tsetse flies in Handeni district and Ngorogoro Conservation Area 4.4. A comparison between Handeni district and Ngorogoro Conservation Area The positive flies in both Handeni district and Ngorogoro Conservation Area. status negative field Handeni Area Ngorogoro Conservation Area Total positive Total 149 7 156 213 6 219 362 13 375 The prevalence of trypanosomes in tsetse flies in Handeni district was 4.49% (7/ 156 * 100). In Ngorogoro Conservation Area the prevalence was 2.73% (6/ 219 * 100). Chi-Square Tests Exact Asymp. Sig. Value Pearson Chi-Square df (2-sided) .831a 1 .362 Continuity Correctionb .391 1 .532 Likelihood Ratio .817 1 .366 Fisher's Exact Test Linear-by-Linear Association N of Valid Cases Exact Sig. (2- Sig. (1sided) sided) .400 .829 1 .264 .363 375 a. 0 cells (.0%) have expected count less than 5. The minimum expected count is 5.41. b. Computed only for a 2x2 table The difference between the prevalence in Handeni district en in Ngorogoro Conservation Area is not significant (p= 0.400 so p › 0.05). 12 The prevalence of trypanosomes in tsetse flies in Handeni district and Ngorogoro Conservation Area 5. Discussion The amount of tsetse flies captured was lower then expected. This means the tsetse density in both areas are lower than expected. This is due to dipping animals every other week in a bath with acaricides. Farmers also use targets. This targets are blue colored screens dipped in insecticides. This appeared to have a positive effect of controlling tsetse flies in both areas. Also increased urbanization, more villages, less game animals and clearing of bushes (by burning whole areas down) for preparation of land for agriculture may have contributed in the reduction of tsetse flies in these areas. The farmers keep their calves around their houses, this leads to a lower tsetse challenge. It could also be a result of the less sensitive diagnostic tools used during the survey or because the survey was during the end of the dry season. Another problem was that the Maasai people living in the area we used to put our traps removed the attractants a few times because of a lack of confidence in our research. This was unexpected for the team, because we put the traps there in consultation with these Maasai people. Both male and female tsetse flies can be infected by trypanosomes and transmit these trypanosomes, but all the positive flies captured in the survey were female. The cause of that is expected to be the fact that female flies live longer than male flies so female flies outnumber the males. Female flies live eight weeks and male flies live four weeks. [7] In Handeni district only T. vivax where isolated. It is a possibility that this is the only trypanosome infecting the tsetse flies in this area. The proboscis of a dissected fly was checked for trypanosomes first. If the proboscis was negative the whole fly was considered negative. This is a possible cause of missing trypanosome infection in midgut and salivary gland. The difference between the prevalences in both areas, 4.49% in Handeni district and 2.73% in Ngorogoro Conservation Area were possibly caused by the difference in altitude of the different areas. Handeni district is 830 meters above sealevel and Ngorogoro Conservation Area is 1700 meters above sealevel. This cause a difference in temperatures between the two areas, which causes different behavior of the tsetse flies. The prevalence of infected cattle in Handeni district was 4.17% and in Ngorogoro Conservation Area is was 1.54%. So there seems to be a correlation between the results from this survey and the prevalence of trypanosomes in cattle. In the survey of trypanosomes in cattle only T. congolense was found. [4]That was a difference from this study, because the dissected flies were also infected with T. vivax. In another survey in Morogoro, Tanzania they found a prevalence of 2.3% of cattle infected with trypanosomes. Also they found T. vivax as the most prevalent spp. in cattle in Morogoro. [9]In Gombe state, Nigeria most cattle was infected with T. vivax. [17] In Gimbi district, Ethiopia was T. congolense the dominant species. In this survey mainly G. morsitans bubmorsitans and G. tachinoides was found. [7]These species were not captured in this study in Handeni district and Ngorogoro Conservation Area. Even with a prevalence of trypanosomiasis in tsetse flies of 4.49% in Handeni district and 2.73% in Ngorogoro Conservation Area this disease causes enormous losses. Costs as increased costs for treatments and control of trypanosomiasis and by poor meat and milk production due to anaemia, immunosuppresion, retarded growth, infertility, abortion, stillbirth and weight loss.[9] Because of the importance of keeping cattle in Tanzania more efforts to control trypanosomosis are needed. In conclusion, despite the use of dip tanks, bush clearing, treatment and targets trypanosomosis is still present in tsetse flies. And because there is a threat of resistance to the trypanocidal drug and the fact that there is no vaccine for trypanosomosis the only solution of controlling this problem is to control the vector population or to use trypanotolerant animals like N’Dama or West African Shorthorrn cattle. These animals have a natural resistance against trypanosomiasis. This means these animals won’t get infected as bad as nontrypanotolerant cattle. A disadvantage of the N’Dama or West African Shorthorn is that they are small animals. Farmers rather want bigger animals because they are worth more, have a higher milk yield and bring in more draught power. [13] Because eradication of the complete tsetse fly population is considered impossible, the aim must be to reduce the population of tsetse flies, so the tsetse fly density will decrease and there will be less contact between tsetse flies and cattle. With intensive surveillance, treatment and control this must be possible. 13 The prevalence of trypanosomes in tsetse flies in Handeni district and Ngorogoro Conservation Area References [1] Anonymous http://insectspedia.blogspot.com/2010/04/tsetse-fly.html,. [2] Anonymous http://www.ilri.org/InfoServ/Webpub/fulldocs/ilrad89/Trypano.htm,. [3] Anonymous www.microbiologybites.com,. [4] Bax E.M., The prevalence of infected cattle by Trypanosoma spp. in Handeni district and Ngorogoro Concervation Area Authority, (2011). [5] Centers for Disease Control and Prevention 1600 Clifton Rd. Atlanta, GA 30333, USA, http://www.cdc.gov/parasites/sleepingsickness/biology.html,. [6] E.A. Wells, THE IMPORTANCE OF MECHANICAL TRANSMISSION IN THE EPIDEMIOLOGY OF NAGANA: A REVIEW, (1972) 2:74. [7] Efrem D.B.1., Yacob H.T.1., Hagos A.T.1., Basu A.K.2., Animal Biology: Bovine trypanosomosis in Gimbi district of Western Oromia, Ethiopia, (2010) Number 2:123-123-131. [8] Guy d'Leteren, Kamau Kimani, Indigenous genetic resources: A sustainable and environmentally friendly option for livestock production in areas at risk from trypanosomes,. [9] H.E. Nonga, D.M. Kambarage, Prevalence of Bovine Trypanosomosis in Morogoro, Tanzania, (2009). [10] Kasilagila G., Studies on trap effectiveness of tsetse flies (Glossina spp. (Diptera: Glossinidae)) in the Tanga Region of north eastern Tanzania, Acta Trop. (2003) 3:385-392. [11] Kristjanson P.M., Swallow B.M., Rowlands G.J., Kruska R.L., de Leeuw P.N., Measuring the costs of African animal trypanosomosis, the potential benefits of control and returns to research, Agricultural Systems (1999) 1:79-98. [12] Malele I., Craske L., Knight C., Ferris V., Njiru Z., Hamilton P., Lehane S., Lehane M., Gibson W., The use of specific and generic primers to identify trypanosome infections of wild tsetse flies in Tanzania by PCR, Infection, Genetics and Evolution (2003) 4:271-279. [13] Mike Lehane, professor of molecular entemology and parasitology. Liverpool school of tropical medicine, http://hstalks.com/main/browse_talk_view.php?t=1853&s=1853&s_id=530&c=252,. [14] Naessens J., Teale A.J., Sileghem M., Identification of mechanisms of natural resistance to African trypanosomiasis in cattle, Vet.Immunol.Immunopathol. (2002) 3-4:187-194. [15] P. van den Bossche, S. de la Roque, G. Hendrickx, J. Bouyer, Trends in parasitology. A changing environment and the epidemiology of tsetse-transmitted livestock trypanosomiasis, (2010) 5:236. [16] R.J. Phelps, D.F. Lovemore, Vector: Tsetse flies, in: Inge du Plessis (Ed.), Infectious diseases of livestock, Oxfort university press, Cape Town, 2004, pp. 43-43-69. [17] Shamaki, B.U.1*, Obaloto, O.B.1, Kalejaiye, J.O.1, Lawani, F.A.G.2, Balak, G.G.1 and Charles D., A wet season survey of animal trypanosomosis in Shongom local government area of Gombe state. Nigeria,. 14 The prevalence of trypanosomes in tsetse flies in Handeni district and Ngorogoro Conservation Area [18] Stéphanie Watier, Etude de l'Infection par Trypanosoma congolense d'un Effectif de Chiens Militaires stationnés en Cote d'Ivoire,. [19] UNESCO world heritage centre, . [20] www.ilri.org/.../fulldocs/LivProd/chapter24.htm, www.ilri.org/.../fulldocs/LivProd/chapter24.htm,. 15