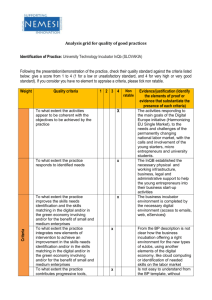
BIP Toolkit
advertisement

Bio-Link IPS 2001-41041 Deliverable D17 – BIP Toolkit COMMISSION OF THE EUROPEAN COMMUNITIES DG ENTERPRISE IPS PROGRAMME BIO-LINK IPS –2001-41041 GENERAL BIOINCUBATION GOOD PRACTICE: An International Review And BEST INCUBATION TOOL-KIT: The Co-Incubation Perspective Philip Cooke* Dan Kaufmann# Avri Havron Naomi Solomon# Robert Wilson* * Centre for Advanced Studies, Cardiff University; # Jerusalem Institute for Israel Studies Incubator Partners: Bio-Link Co-ordinator, Nigel Wild, Oxfordshire Biotechnet Ltd; Rafi Hofstein, Hadasit Bioincubator, Jerusalem; Gabriel Mergui, Genopole, Evry-Paris;Valter Songini, Consorzio Ventuno, Sardinia; Verena Trenker, BioM, Munich February 2005 2 Contents Page Global Bioincubation Good Practice: An International Review 1. Introduction..........................................................................................................................4 2. The Importance of Seeing Bioincubation as a Key Part of Life Sciences Innovation...5 3. Comparing Eight Bioincubators By KVC Services and Governance.............................6 4. Assessing BioIncubator Services Provision and Management Good Practice.............17 5.Good Bioincubation Management Practices Outside the Bio-Link Targets..................22 6. Management Practices That Contribute Most To Successful Performance.................23 7. Bioincubators and Incumbent Firms Being Regionally Embedded...............................25 8. Challenges for Bioincubators.............................................................................................26 9. Conclusions & Recommendations.....................................................................................32 Best Incubation Tool-Kit……………………..……………………………………………..34 3 1. Introduction 1.1 This report is concerned with incubation of start-up businesses in biotechnology, and specifically whether there are prospects for co-incubation of start-ups through international incubator linkages. It asks three key questions about the problems of transforming basic, research knowledge into commercialised products and services in biotechnology. First, we inquire as to whether the span of knowledge control required varies according to regional setting (Cooke, et al., 2003)? We further ask about the knowledge : institutions balance in the context of incubator firm commercialisation capabilities. Second, it asks further if incubation is the only or best general method for enhancing exploitation of basic research and assesses alternative means. Third, we ask to what extent the incubation process, located at the higher risk end of the commercialisation continuum, is necessarily a non-profitable, publiclysubsidised service and whether regional location influences the subsidy requirement unduly. The data enabling us to answer those questions in our conclusions were generated during the first and second stages of an EU project (Bio-Link)conducted during the first and last three months of 2003. Further data were mobilised from a survey of bioincubators outside the BioLink targets. 1.2 The assessment of Bio-Link incubators involved researching five European and Israeli incubators in the first stage and comparing them with three North American ones in the second stage. Then accessing further secondary data on bioincubation elsewhere. The resulting analysis provides detailed presentation, monitoring, assessment and evaluation of incubators by type, by service offered, by ownership and by position in what can be called the knowledge value chain for Life Sciences more generally, and biotechnology more particularly. In biotechnology, this value chain passes from exploration through examination to exploitation knowledge as basic research evolves to the stage of Pre-Clinical and Clinical Trials and then to the Commercialisation stage where due diligence skills and venture capital skills ultimately determine whether or not a ‘prospected’ start-up may reach the stock market, license its technology or be acquired by another owner. 1.3 Following this brief introduction and following exposition, the paper then moves into the main stage of comparing and contrasting, monitoring and evaluating the eight incubators in the study, recording the judgements of both academic observers and incubator managers regarding their specific incubator type, strengths and challenges, or preferred market position. An attempt is made to resolve these judgements where variation occurs. There is always an attempt both to situate the bio-incubators in the wider local or regional innovation system 4 (Cooke, 2001) within which they reside, and to recognise that where one may offer a wider range of services to academic entrepreneurs, this may be a sign of system weakness rather than strength, although for other reasons – ‘the one-stop shop’ – it may of course be a sign of efficient focusing of many support functions under one roof (Wolter, 2002: Cooke, 2002). Finally these results are compared with a further survey conducted in support of Bio-Link by Cardiff’s Centre for Advanced Studies on bioincubator management from beyond the BioLink targets. 2. The Importance of Seeing Bioincubation as a Key Part of Life Sciences Innovation 2.1 The role of incubation in the Life Sciences knowledge value chain on the one hand, and in knitting together exploration, examination and exploitation services to start-ups and evolving businesses, on the other, is clearly crucial. In both North America and Europe, influential reports have recently been published urging incubation upon policy-makers and markets. In the US case they were seen as exerting a positive effect on growth in new technology sectors generally though this was less clear for biotechnology incubators benchmarked (NBIA, 2002). In a UK report ( CSES, 2003) focused on bioincubation, 22 facilities were examined, were found expensive at £4.6 million average running costs per year but likely to grow in demand for space in the near future. 2.2 From an intellectual viewpoint we may simply list the kind of ‘boundary-crossing’ services of value to spinout biotechnology businesses according to the span across the ‘3 E’s’ we consider our eight bioincubators to cover in varying degrees: In relation to Exploration – incubators co-located or otherwise in reasonable proximity to basic research institutes may offer services related to assisting transforming the germ of a business idea into a business. Proof of Concept, validation and selection of Exploration candidates for possible incubation by virtue of ‘prospecting’ and technological ‘due diligence’ may characterise this variant of an Examination service. This occurs through network knowledge of scientific or technological and financial expertise, knowledge of sources of seedcorn finance, or personnel and sources of public funding, training or advice. In relation to Examination – apart from the functions noted above, bioincubation will involve advising and servicing requirements start-up firms that have passed beyond Proof of Concept to Pre-Clinical Trials may need regarding the administration of such trials. These may involve CROs, large pharmaceuticals firms or other means of mobilising appropriate mammalian testing resources. Other forms of testing of 5 diagnostic kits or biomedical devices will also be provided directly, or indirectly through the bioincubator management’s trust-based ‘social capital’ In relation to Exploitation - equity-based syndication conducted by investors may be mobilised by incubator management, strategic partnering, joint ventures of a technological nature and connections to consultants, specialist lawyers and IPR advisers and management accountants for business ‘engineering’ will be supplied inhouse or through the social capital networks that accrue to bioincubators and constitutes their key management resource of value along with the capability to supply appropriate ‘wet lab’ space. Relationships with markets are especially important in assisting firms to find expert commercial partners (Norton, 2000; Zook, 2000: Cooke, 2002; Powell et al., 2002). In section 3, efforts will be made to characterise the eight bioincubators in the Bio-Link study according to the range of services they supply and the direct and indirect nature of those services. 3. Comparing Eight Bioincubators By KVC Services and Governance 3.1Rationale for Incubator Selection 3.1.1 A first question is to inquire about selection of bioincubators for study. Given this is a policy research project funded by DG Enterprise of the EU, policy concerns are naturally foremost. Thus from Europe and Israel (a signatory and financing member of EU RTD programmes), the range of funding and governance forms, from public to private was sought. Moreover, those in prosperous and less prosperous regions were of interest, as were any distinctive characteristic specialisation in services provided. Hence, two heavily subsidised public bioincubators, one in the prosperous Paris region of France, the other in the less favoured Sardinia region of Italy were selected. Moreover, the former was considering how to offer seedcorn funding to a wide range of tenants while the latter offered newly built bioincubator property with access to the unique Sardinian gene bank for bioscientific and genomics research firms. By 2004, this had already attracted firms from such locations as Milan, seeking to access Sardinian regional funds to assist expansion. 3.1.2 A second group of bioincubators were privately funded, albeit by private foundation rather than solely by market transactions. These are located in prosperous Oxford, England and less favoured Jerusalem in Israel. The Oxford bioincubator is surrounded by a rich 6 infrastructure of exploration, examination and exploitation knowledge services ranging from Oxford University life sciences and medical research, to Oxford University’s celebrated Isis Knowledge Transfer Office (KTO), and Clinical Research Organisations (CROs) to regional public and private venture capital funds. The Israeli bioincubator is the heart of a small, dynamic biotechnology cluster with the internationally renowned Hebrew University and related teaching hospitals nearby, the former one of the world’s richest patent income earners, at third with 2002 income of over $40 million resulting from the invention in its biosciences faculty of the globally-consumed ‘cherry tomato’. This incubator provides many services others do not, notably IPR services and early stage funding. However, for it, the idea and prospect of international and even global networking through bioincubator nodes abroad acting as gatekeepers to desired services is highly attractive. 3.1.3 The third European bioincubator ‘system’ is that presided over in Munich, Germany by the public-private partnership funding and exploitation agency BioM, winner of the prize as one of Germany’s main biotechnology clusters under the BioRegio programme that ran from 1995-2002 and resulted in Munich becoming Germany’s main biotechnology cluster location. Hence, such a public-private funding partnership was intellectually interesting in bridging the gap between largely or wholly public support as in France and the rest of Germany, and the private, foundation support found in Britain and Israel. Moreover, BioM offers a variety of support services for new biotechnology firms, including assistance in accessing space at the bioincubators in Martinsried, the south-western Munich suburb where a major bioscientific research and commercialisation ‘megacentre’ has evolved, albeit relatively recently with respect to spinout activity. 3.1.4 Next, staying with the governance, funding and regional service profiles as selection criteria, the following North American bioincubators were selected for examination. The first is a mainly public sector supported facility in Quebec, Canada based near Montreal on the University campus at Laval. The Quebec BioIncubator Centre (QBIC) is part of a large Biosciences innovation complex and comparable in philosophy to Genopole. Montreal and environs has experienced significant industrial restructuring, loss of traditional manufacturing industry, and particularly financial services to Toronto during the 1970-2000 period. However, bioscientific research is deeply embedded in the region and commercialisation has produced world-class firms, notably and also at the Laval campus, BioChem Pharma discoverer of Epivir, the first AIDS treatment marketed through Glaxo. Since acquired by UK firm Shire Pharmaceuticals, BioChem was, in late 2003, experiencing significant corporate re- 7 positioning towards less costly therapeutic targets while advanced research was being focused more towards home base. 3.1.5 The two bioincubators selected in the USA were, first, the Broad Hollow Bioscience Park, at Farmingdale, Long Island, New York, part of the State University of New York campus at Farmingdale and on the doorstep of the Cold Spring Harbour Research Institute, headed by James Watson, co-discoverer of DNA and co-initiator of the Human Genome project. The building of this bioincubator was funded by the state of New York, SUNY Farmingdale and the support of Cold Spring Harbour. The incubator’s running costs are largely met by Cold Spring Harbour spinout OSI Pharmaceuticals (acquirer of British Biotech in 2003), a successful business employing 120 and occupying most of the incubator. OSI was gifted one of Pfizer’s R&D projects for an Oncology drug on monopoly legal grounds consequent upon their acquisition, also in 2003, of Pharmacia, possessor of its own Oncology treatment. Hence, while not a public-private partnership, this incubator receives private maintenance and related services from the private sector. 3.1.6 The second American bioincubator in the comparison is Massachusetts Biomedical Initiatives and specifically its MBIdeas (MBI) Centre at Winthrop St., Worcester, Massachusetts. Massachusetts Biomedical Initiatives (MBI), established in 1985 is an independent, tax-exempt corporation created to support the growth and expansion of biotechnology and medical device companies throughout the region. The set-up funding for the MBI facility came from a grant of $1 million from US Federal level through the Economic Development Administration (EDA) of the Department of Commerce. This was matched by a $1 million of private sector funding and $1 million from the State of Massachusetts. The healthy financial model at MBI is underlined by the fact that the majority of its operating income (80%) for the Worcester facility comes from rents. The remainder came from the State of Massachusetts in the form of grants, with the latter portion of income falling gradually. The intention was for further space to be bought in the former St. Francis Hospital that houses MBI. With expansion, the bioincubator would become self-funding. Hence MBI is the nearest to a non-foundation, private bioincubator operating in the market with low public funding. However, in essence, its funds come from rents and will come from a 1% equity investment from incubator tenants. Hence it is becoming a market-facing institution unlike the other incubators in this research study. 3.1.7 Thus a degree of complexity underpins the precise choices made. Key determining criteria were the nature of funding, extent of institutional support from agencies or market 8 services available nearby, nature of scientific research and exploitable knowledge, receptivity of scientists towards academic entrepreneurship, and rigour of spinout screening. Each bioincubator illustrates in distinctive ways strengths and weaknesses in this set of criteria. Hereafter we will take account of the observed position of each bioincubator in the range of elements for which it takes responsibility from the Knowledge Value Chain (KVC) and illustrate a governance model that characterises each of our eight incubators. This will be attempted in Fig. 4.1 by deploying a two-by-two schema arranging governance on the ‘x’ axis and KVC on the ‘y’. This follows brief thematic accounts of each bioincubator. 3.2 The Trust-Funded Incubators 3.2.1 These are Oxford BiotechNet and Hadasit in Jerusalem The former is a ‘virtual’ incubator networking some 70 ‘mentors’ available to sell, at or below market rate, market services to start-up tenants in hard accommodation owned by the bioincubator. In turn this is an affiliate of the Oxford Trust, a charitable foundation, itself arising from the profitable activity of Oxford Instruments, a successful scientific instrumentation business. To help set up the bioincubator a € 600,000 grant was won under a UK government (DTI) ‘Bio-Challenge’ scheme in 1997 which assisted investment in incubator buildings. Although initial plans were for 1,000 square metres at the Churchill Hospital, land was found at the former site of Yamanouchi Research, the Oxford home of Japan’s third largest pharmaceuticals business. Other shareholders invested nearly double the ‘Challenge’ funding and space was opened in mid-1999. Oxford is one of the UK’s leading biotechnology ‘clusters’ with some fifty core biotechnology businesses (in the early 1990s only 3 or 4) and a further seventy support firms, located at various sites, including along the A34 ‘corridor’ to Abingdon. 3.2.2 Some are spin-outs from Oxford University Life Sciences and Medical School centres and departments. The Oxford incubator has 12 tenants, mostly single person companies moving towards second or third phase development. All are in biopharmaceuticals, ranging from reagents, to therapeutic sugars, gene therapy, cancer therapy, antibodies and bioinstrumentation. Most have UK innovation awards, following exhaustion of which if Proof of Concept is validated, business angel funding is intended to lead to product sales after some five years. One successful firm associated with the bioincubator is Oxford Glycosciences, in 2003 acquired by Celltech, itself acquired by UCB, the Belgian pharmaceuticals and chemicals group in 2004 as the UK’s biotechnology sector began to consolidate. Thus BiotechNet is a private, not state-funded facility, though it has clearly benefited from state setup funding. It lacks either the power or responsibility to seek a return for incubator services beyond rent. It provides market network access to private equity linking to, among others, 9 Oxfordshire’s University Enterprise Network. Some firms coming to the bioincubator are thoroughly vetted and validated by Oxford University’s Isis commercial office. Its strengths are its reputation, its image as a model to other incubators, its uniqueness in Oxfordshire and its strong university and mentoring links. Its weaknesses are small size, with only three staff, and absence of a seed fund of its own. Future plans are to grow and offer ‘accelerator’ and ‘follow-on’ space. It is anticipated that the Yamanouchi site may in future host a Science Park which may meet such aspirations. 3.2.3 Hadasit is fundamentally a for-profit, incorporated company founded by the Hadassah Medical Organisation (HMO) , a women’s health foundation that owns 100% of Hadasit’s shares. It is unlike the Oxford bioincubator as it incorporates the Knowledge Transfer Organisation (KTO) function, generating a royalty stream from its investment in spin-offs (see also the MBI account below). In this way, it offers a more comprehensive, one-stop-shop service. Hadasit’s aim is to increase the revenue base of the incubator. Its procedure involves screening firm candidates, agreement for pre-Proof of Concept funds, followed by an IPR assessment. If it passes muster, a patent filing occurs conducted by Hadasit, leading to a final prototype, preparation of a business plan and auditioning for venture capital. Hadasit has US partners and also links to incubation facilities in Singapore and Australia. It offers firms the widest range of services and benefits from growth in its equity stake in incubated start-ups. The incubator has firms specialising in thrombosis, cancer care, rheumatoid arthritis and hormone research. 3.2.4 The key differences between these similarly originating incubators is that Oxford has a strong knowledge and institutional base, hence the incubator is a waystation for well-screened candidates from the Isis spinout system. It is profitable because of high occupancy levels. Hadasit is a one-stop shop in a country that despite its small size, is rich in knowledge but would like to expand its institutional environment. Israel has an extremely well-developed and sophisticated technological incubator system andis a country that punches well above its weight. Hadasit generates an equity-based income stream to augment rental income, but payback times are such that new business models that speed up investment engagement are actively being assessed. 3.3. The Public Subsidy Bioincubators 3.3.1 Genopole is member of a French network of such incubators with Evry (Paris) as the leader. BioM is a public-private partnership, a private company but with income mainly derived from public sources, promoting biotechnology entrepreneurship in Munich. Consorzio 10 Ventuno is a genomics technopole funded by the Sardinian regional government. Genopole’s ‘family’ are in Lille, Rennes-Nantes and Strasbourg. Genopole was established in 1998 as a public agency with a mission ‘to create a new BioPark association between public sector and industrial research’. Organisations like CNS (gene sequencing), CNG (genetics) and Genplante occupy space amongst public research bodies like CNRS, INSERM, CEA, INRA, and Evry University – employing 1,520 people in all. Research focii are gene analysis, gene function, gene therapy, nanobiotechnology and Life Sciences research. 3.3.2 Its strengths are its capabilities to foster ‘boundary-crossing’ interactions between public, private and university research and exploitation. Weaknesses are the difficulty of ‘prospecting’, as few start-ups volunteer to be so and lack of seed stage funding, even for Proof of Concept support. The Genopole procedure is to Prospect, then following Detection, Sign a Protocol for a Prevalidation Strategy for a spin-out firm. Thereafter progress is repeatedly validated by an Audit Board leading, if positive, to Shareholder agreements, Preseed funding (mostly grants), and a Task Force assisting Project Management towards a Business Plan presented to Venture Capitalists. This will include ANVAR, the French state venture fund. The aim is to access €1 of grant matched with €1 of equity. The EU Fund of Funds (venture capital) brings 25% of such funding. In 1998 there were 10 biotechnology businesses employing 331 persons, in 2000, 34 employed 561 and in 2002, 41 employed 602 persons. There are knowledge spillovers in Genopole, partly orchestrated by open seminars for academics and entrepreneurs. Club Genopole meets every 4/5 weeks and Les Doctorials are free days when all future doctoral students learn entrepreneurship. 3.3.3 In Munich, Martinsried in the south western suburbs marks the centre of biotechnology research and incubation in Bavaria. The Biotechnology Innovation Centre (IZB) financed with €50 million by the Bavarian government is located in Martinsried with over 14,000 square metres of laboratory and office space. The organization responsible for managing development of biotechnology, BioM, is also located in the IZB. The area has become a biomedical research campus with researchers working in biology, medicine, chemistry and pharmacy located there. BioM AG is a one-stop shop with networking, public relations, seed financing and enterprise support under one roof. Seed financing is a mix of BioM funding from their equity plus a maximum contribution from the Bavarian government of 25%. The maximum amount of funding per company is normally €250,000. By 2003, numerous startups had been funded to the tune of €120 million. DBFs increased from 36 to 120 between 1996 and 2001 (Kaiser, 2003). However, in 2004 some 6 of 28 bioincubator start-ups were forced into bankruptcyby the biotechnology investment downturn, leaving in 2005 only 22. 11 BioM is a network organization, reliant on science, finance and industry expertise for its support committees. It also runs young entrepreneur initiatives, including development of business ideas into business plans and financial plans. Business plan competitions are also run in Munich. 3.3.4 Sardinia’s regional innovation policy promotes clusters and knowledge economy development through science-based industry, R&D and incubator activity. Pharmacogenomics is one of five clusters in development. In pharmacogenomics, Sardinia’s uniqueness in reduced genetic diversity from its long-settled population, especially in the interior, facilitates research and commercialisation in endogamy and consanguinity. There is a strong knowledge base and genetic skills in firms and institutes like SharDNA, Neuroscienze, CRS4 (the Centre for Advanced Studies, with founding chairman physics Nobel laureate, Carlo Rubbia) and Parco Genos. The incubator has R&D facilities for research into genes with multigenetic disease as targets. The Gene Park has 13 biotechnology firms and research institutes. A recent arrival from Milan is Bio-Ker, a firm engaged in recombinant therapeutic proteins, biopharmaceuticals, DNA vaccines and recombinant enzymes. 3.3.5 Although firms and research institutes specialise, in many instances, in work related to Sardinia’s distinctive, genetically undisturbed population, a problem regarding spin-out concerns the reluctance of the local university to work with the research organisations (such as STP). Accordingly, no spin-off occurs from university incubator interactions, despite the fact that the University of Cagliari, for example, specialises in genetics, biology, neuroscience and toxicology. Hence firms may come into being by non-academic routes. SharDNA, for instance, arose from research conducted at the CNR Molecular Genetics Institute and funds from Renate Soru, founder of Internet giant, Tiscali based in Cagliari. State involvement is crucial to the existence of the Consorzio Ventuno bio-incubator, as in France. Recently the Sardinian regional government built an animal house for Neuroscienze an indigenous and there is a regional R&D investment fund for research projects. 3.3.6 Of these, Munich was belatedly, following the success of BioRegio, the most successful generator of new biotechnology firms although many were close to bankruptcy after the downturn in financial markets, the reluctance of VCs to continue investing in early stage companies and the BioRegio public subsidy declined with the ending of the programme. As we have seen, 6 start-up biotechnology businesses became bankrupt in 2004 alone. Strength in the knowledge base was augmented by network partnership among funding channels and enterprise support agencies to create what were once 28 at the peak. Consorzio Ventuno is 12 weak because of thin capabilities in knowledge exploration and exploitation. Genopole has a full range of mainly public support services with a moderately good knowledge base but weak commercialisation capabilities due to a weak ‘deal flow’. 3.4 Subsidised Bioincubators in North America 3.4.1 Each of the three bioincubator comparators from North America is publicly subsidised but this varies as a proportion of total costs to as little as 20% and potentially declining in MBI Massachusetts. The most ‘European’ is the Quebec Biotechnology Innovation Centre (QBIC), inaugurated in May 1996. It was created to promote the launch and development of biotech companies specializing in health, environment, agro-food and forestry industries in the greater Montreal region. The region has a large biomedical cluster with leading companies and a strong research base with four universities in Montreal, an established biopharmaceuticals industry with 145 companies, 14,500 jobs and 50 biological research institutes including the Canadian National Research Council Biotechnology Research Institute, an important federal biotechnology research centre. QBIC is located in the Laval Science and High Technology Park, Montreal. The Park was created in 1989 as the result of a strategic alliance between the INRS-Institut Armand-Frappier, (a research centre of Quebec University) the City of Laval and Laval Technopole. The Laval Science and High Technology Park is the focus of “Biotech City”, a $100 million initiative launched in June 2001 to develop a business and science centre supported by the Quebec government, Investissement Québec, the Institut National de la Recherche Scientifique (INRS), the Laval Technopole and the City of Laval. Some 30 businesses, biotechnology and biopharmaceutical companies, research centres and IT firms exist in Biotech City. 3.4.2 QBIC had, in late 2003, six firms in its bioincubator. It is a not-for-profit organisation with two funding partners: first, the INRS-Armand-Frappier Institute of Immunology and Virology at the University of Quebec, and second, Laval Technopole, which is the Economic Development Agency of Laval City. QBIC relies on the support of the Canadian and Quebec governments for around 45% of operating finance with the remainder self-financed through mainly rent (c. 30%) and other services such as the hire of scientific equipment to outside companies. Private sector sponsors such as the Royal Bank of Canada – the main commercial bank in Canada – have also invested. 3.4.3 As with Genopole, the Montreal industry required attention at the pre-incubation as well as incubation stage. Pre-incubation is usually a 6-9 months period which includes help in writing a business plan, negotiations over the intellectual property rights and looking for the 13 first round of investment. Selection of start-ups at the pre-incubation stage is based on the technology alone but the process of incubation puts QBIC in a strong position to evaluate potential incubator clients. This is followed by an incubation stage of 2-3 years during which the company builds its value and brings its technology to a point where it can be successfully commercialised and the company can become independent. QBIC has a wide range of incubation periods, from 3-6 months to 3-4 years with many companies staying the full four years. The Centre considers between 15 and 20 proposals for start-ups per year with around 3 accepted. 3.4.4 To ensure that it admits only scientifically and commercially viable companies, the Centre (like Genopole) carefully screens all applicants. A selection committee evaluates candidates according to the following criteria and general guidelines: the company must operate in the biotechnology sector or health industry; demonstrate that its activities result in technological advances; and demonstrate that it has the necessary technologies and financial resources to execute its business plan. The selection procedure takes two weeks and is evaluated by two scientists, two financial experts and the QBIC manager before being referred to QBIC’s Executive Committee for a decision. To screen companies, QBIC may use external consultants such as other biotechnology incubators in Montreal. The incubator entrepreneur has the opportunity to choose one of the consultants. If a company is accepted, the technical director will ascertain their operational needs in discussion with the clients by using a customised database capable of determining the clients’ needs in terms of laboratory equipment and layout. 3.4.5 Start-ups at the pre-incubation stage can apply to QBIC’s seedcorn fund which is a rotating interest-free unsecured loan up to a maximum of 75% of projected costs repayable in full when companies secure second round funding. The remaining 25% of finance must be raised by entrepreneurs, with regional funds or institutional venture funds the main sources. A business mentoring service is available to help reduce start-up costs and equip companies with appropriate human and financial resources. The mentoring structure relies on the bioincubator manager who acts as a first-line advisor to the heads of resident companies. Further mentoring expertise is provided by a network comprising consultants covering the seven major fields of bioincubation competence and acting as an advisory committee. 3.4.6 QBIC occupies 27,000 square feet of space including 20 wet laboratories and 19 offices, its 2003 enrolment was 6 resident companies, with 13 graduate companies having moved on to other premises. Start-up companies have access to facilities such as laboratories, offices, 14 specialised storage areas and basic furniture. Tenant companies also have access to a host of sophisticated scientific instruments on a time-sharing basis for no extra cost. A key attraction of QBIC to clients is the wide array of equipment available so clients have to buy less equipment themselves, leaving funds to pay for research staff. The broad flexibility in equipment usage is also a key asset of QBIC. Clients are able to use equipment in their own laboratories as well as in the common areas, alleviating security concerns for some clients. The graduation policy at QBIC entails writing a business plan 12 months prior to graduation, raising a final round of financing, and setting a time limit on occupation at QBIC. Part of the graduation policy stipulates that companies are not allowed to grow to more than 25% of the total incubator space. A large majority of companies (90%) graduating from QBIC have been successful in raising capital after incubation with the average amount raised around $120 million. Two companies graduating from QBIC are now listed on the CDNX – the Canadian small-capitalised stock exchange. Hence, the French approach to nurturing spinout firms from pre-incubation to graduation is as pronounced at QBIC as at Genopole, although the need for ‘prospecting’ is less acute in Montreal. This is because there is a stronger knowledge, institutional and market base for biotechnology there than in Paris, although subsidy is vital for bioincubation in both. 3.4.7 Broad Hollow Bioscience Park (BHBP) is a not-for-profit biotechnology research park joint venture between SUNY Farmingdale and Cold Spring Harbour Laboratory (CSHL). The aim of the Park is to establish a bioincubator facility to sustain economic development in the region by attracting public and private funds to further exploration, examination and exploitation knowledge capabilities. A cluster and associated jobs are envisaged in the regional economic development strategy centred upon CSHL , an internationally renowned research and educational institution specialising in research on cancer, neurobiology, plant genetics, genomics and bioinformatics. 3.4.8 BHBP has a $15 million incubator facility, opened in September 2000 with the first building of 63,500 sq. ft. located on a 20-acre section of the Farmingdale campus. In September 2002, New York State support of $20 million was announced to construct a second building of 50,000 sq. ft. for start-up biotech companies. The incubator houses the research headquarters of OSI Pharmaceuticals Inc., the anchor tenant, employing 120 people and occupying 85% of the incubator, and two start-up companies, Helicon Therapeutics Inc., a further spin-out from Cold Spring Harbour Laboratories and Immuno-Rx, Inc which together employ around 35 people. 15 3.4.9 BHBP supports the development of biotechnology start-up companies by providing shared facility resources, and the resources of the Farmingdale State campus, partnering with surrounding businesses and research institutions. This supports a strategy for growing biotechnology cluster companies along Route 110 Bioscience Corridor. The latter is a regeneration partnership between the public, private and civic sectors, and two Long Island Towns, Huntington and Babylon. It was established in 2001 to enhance the future development of the Route 110 corridor as Long Island’s economic hub by attracting high-tech firms. Efforts are focused on improving the business environment and infrastructure. A group called the Long Island Bioscience Association promotes the region as a bioscience corridor. 3.4.10 Massachusetts Biomedical Initiatives (MBI) was established in 1985 as an independent, tax-exempt corporation created to support the growth and expansion of biotechnology and medical device companies throughout the State. One of MBI’s main initiatives is the MBIdeas Innovation Centres, incubation facilities designed to nurture start-up biomedical companies. Since then, MBI and its former venture capital creation, Commonwealth BioVentures Inc. (CBI), have invested over $8 million of public funding with over $50 million of private money in new technology driven companies, and have developed two major new Innovation Centres. These have helped to create some 20 companies. Over time, firms historically receiving support from the MBI/CBI alliance have employed over 2,000 people, 1,500 located near Worcester. These companies now have over $50 million a year in payroll and they have raised $600 million of additional finance, fuelling the economic growth of the region. MBI has served as a national model for leveraging public sector funds with private sector investments to promote economic development. Most of this funding came in the past from federal sources. The operating budget of MBI has grown from less than $500,000 in 1985 to over $2 million in 2003. 3.4.11 The healthy financial model at MBI is underlined by the fact that the majority of operating income (80%) for the facility comes from rents with the remainder coming from the State of Massachusetts in the form of grants, with the latter portion of income falling gradually. It is a strong aim of the CEO at MBI for the facility to become self-funded. A further source of income is from MBI’s policy to take a 1% non-dilutable equity stake in their start-ups, a policy that should soon reap benefits with two of its graduate companies currently going public. 3.4.12 In late 2003, MBI had 12 start-up product and service companies and the performance of its graduate companies was notable with only two out of 20 graduate companies failing to 16 raise any capital after incubation. On average, start-ups at MBI raise $250,000 from ‘Friends, Family and Fools’ (FFF) referring to the social capital finance sources that start-ups use in the absence of seedcorn funding, which is unavailable at MBI. Upon graduating from MBI, companies have raised on average $50 million of further funding with one company, ViaCell Inc. a stem cell company, aiming at becoming a quoted company with $120 million of finance. The majority of companies at MBI originate from the two local institutions, the University of Massachusetts Medical School and Worcester Polytechnic Institute, although some come from MIT and from large organizations such as Genzyme, Pfizer and Waters, a local pharmaceuticals firm. 3.4.13 To conclude this profiling section, it is instructive that all bioincubators are subsidised by public infrastructure and running cost budgets, MBI being the nearest to self-sufficiency. It is clear that the harder the spinout process is, the more the incubator is expected to supply services that are available elsewhere on the market. Exploration knowledge capabilities vary and within a narrowed frame of potential spinout activity, ‘prospecting’ is necessary and often unrewarding or impossible. The attractions of co-incubation are clearly greater for those bioincubators lacking surrounding capabilities in key elements of the knowledge value chain. For those like Oxford BiotechNet and MBI, the abundance of exploitable knowledge and available examination and exploitation services means they are only modestly engaged in other than activities to do with running the incubator and collecting rents. 4. Assessing BioIncubator Services Provision and Management Good Practice 4.1 From the typology analysis provided, it may be seen that each incubator is distinctive but that they may be categorised usefully in two ways, first by characterising their governance 17 Public Governance Mixed Governance Private Governance Value Chain Consorzio. Genopole. QBIC BioM. BHBP Hadasit MBI BioTechNet Genetic Assets Yes No No No No No No No Research Yes Infrastructure Yes Yes No Yes Yes No No Project Selection No Yes Yes Yes Yes Yes Yes Yes Funding No Yes Yes Yes No Yes No No Validation No Yes Yes Yes No Yes No Yes Incubator No Yes Yes No Yes Yes Yes Yes Patenting & Licensing No No No No No Yes No No Patenting Advice Yes Yes No Yes Yes Yes Yes Yes General Business Advice Yes Yes Yes Yes Yes Yes Yes Yes Exit Advice No Yes Yes Yes Yes Yes Yes Yes Fig .4 1: Bioincubator Assessments through Academic Research Team Monitoring models and, second by considering the extent incubator services cover the range of knowledge value chain activities. The latter can be divided into ten stages and the former into three types – public, mixed and private – relating to governance, funding and degree of reliance on state subsidy. Fig 4.1 attempts to classify each incubator accordingly. 4.2 Clearly, there are differences, particularly between Consorzio Ventuno and the rest. It could be said (in the Monitoring Assessors’ judgement) that it is not really an incubator, but rather a Science Park with incubator aspirations, in 2004 fulfilled with Bio-Ker attracted. Hadasit, closely followed by Genopole, have the fullest range of services on offer through the knowledge value chain. While BiotechNet and BioM offer the least. However both are surrounded by many such services ranging from a strong external research infrastructure (but 18 not in their premises). And networks for providing patenting and finance. In BiotechNet’s case, this extends to some initial project (firm) selection which is conducted by Isis at Oxford University. 4.3 BioM is largely a key node in a biotechnology services network, and through its committees funds spin-out businesses. BioM links to external infrastructure, such as the Martinsried bioincubator facilities. The KVC gap that BioM plugs is principally seedcorn and other funding for start-up bio-businesses. Generally, there is little variation according to whether incubators are funded publicly or by foundation, Hadasit being notable for the many active and direct services it provides. 4.4 In North America, as we have seen, QBIC is comparable to France’s Genopole but with aspirations to be independent of public funding, with an income stream from hiring out campus-wide equipment, but relatively few tenant companies. This is something noteworthy from the transatlantic comparison, namely that the range of firms per bioincubator is comparable at between 12 and 3. Broad Hollow is dependent on rental income on the basis of up-front public sector investment aimed at ‘cluster-building’ in biotechnology. The presence of Cold Spring Harbour Laboratory is a guarantee of interesting spinout firms and with New York city on the doorstep spinout funding is not difficult if firms are outstanding candidates. MBI is relaxed about accepting incubator firms. They need only a business plan, IPR and a valid product on which to work and if there is no queue, they gain access. While QBIC has a seedcorn fund, the Americans rely on the ‘Golden Rolodex’ of business network contacts to link firms to venture capital and other services, firms themselves typically arrive with more seedcorn funding from FFF sources than is the case in Europe or Israel. 4.5 We now come to the rankings made by incubator managers of their perceptions of what is most important about the services they offer. The main areas of difference between their SelfAssessment and the Academic Monitoring Assessment refer to research infrastructure, which BiotechNet rates highest in its assets. This must refer to Oxford’s surrounding research infrastructure rather than the Oxford bio-incubator premises. ‘Prospecting’ was introduced as a category of incubator knowledge activity. Interestingly, none feel they do it well, particularly Genopole with which it is charged. On selection, Genopole is quite rigorous and considers that its premium service. Consorzio 21 confirms it does modestly or worse for many core incubator services. Interestingly, all four incubators ranked their ‘exit-strategy’ services very low, this being classically a risk-facing activity for which venture capitalists have the appropriate skills. Mentoring was also added to Fig. 4.2’s tabulation and managers felt they were either moderately good or moderately poor at supplying that service with no governance 19 emphasis explaining it. On balance and with a few exceptions, the Self-assessments did not diverge massively from the Monitoring Assessments. Factors enabling value creation Bio-environment assets Basic research infrastructure Entrepreneurship Prospecting Selection Technology validation Access to physical infrastructure Mentoring Internal IP services Access to funding Networking Assistance with “exit” strategy Consorzio Genopole 21 1 6 Hadasit QBIC BHBP MBI OBTN BioM 12 12 12 12 12 12 3 2 1 1 1 7 1 3 9 8 10 11 7 12 1 10 2 6 3 11 11 10 6 9 4 11 9 4 12 11 8 2 9 11 6 2 11 4 6 2 5 7 2 7 3 3 8 6 5 4 11 8 9 8 7 6 8 6 9 4 8 7 9 4 3 4 3 3 5 7 1 7 12 8 9 5 10 4 5 2 5 1 2 5 10 5 10 1=High 12=Low Fig. 4.2: Incubator Manger Self-Assessments of Service Priority 4.6 In the North American cases, they offer principally a property venture in which good candidate firms can find a home. They each offer equipment and even wider access to campus equipment in two cases. None offers important in-house services, although QBIC has a seedfund. Rather, each relies on network contacts, like Oxford’s BiotechNet to link firms to specialist market services of consequence to commercialisation. There is remarkably little selection effort compared to the French and German cases, in particular. Rather, it is known good spinouts exist, also that resources may be found in the regional community. Hence bioincubators, clearly in the MBI case, rely on self-selection whereby a firm arrives armed with acceptable business plan, IPR and a product or service in view, and if space is available, they enter the bioincubator on the assumption that within eighteen months they will have graduated to larger premises elsewhere. In North America, too, bioincubator managers are all businessmen not scientists, whereas in Europe and Israel the reverse tends to be the case. 4.7 An issue arises from this comparison, based on the small numbers of firms hosted in bioincubators everywhere and the great length of the process whereby knowledge moves from 20 exploration to exploitation phases. This is that incubation may not be the most efficient way to bring products and services to market since the discovering firm is itself less than optimally efficient. An alternative model where knowledgeable venture capitalists spot excellent candidate products at Phase 1 stage (in the laboratory or incubator) then take them forward through Phase 2 with modest patient trialling by outsourcing to CROs, then sell on the strongest candidates to pharmaceuticals firms to do Phase 3 trialling and, if successful, marketing and distribution, might be a more efficient model. BioLine, a Hadasit incubator firm, performs this innovative function. Moreover, the view was expressed at Bio-Link’s Evry seminar in February 2005 by a senior representative of France’s leading biotechnology venture capital company, Sofinnova Partners, also located in San Francisco, that good candidate firms were nowadays well-advised to consider forming partnerships direct with pharmaceuticals firms keen to license good innovations or even acquire the company. So much has the 1990s venture capital model declined into risk-aversity that bioincubation must begin to take such observations seriously into account. However, for the moment, incubation remains the hegemonic exploitation (commercialisation) knowledge-transfer model, leading hopefully to venture capital equity investment and IPO, on both sides of the Atlantic. 4.8 Thus we have seen how the knowledge value chain in Life Sciences is extremely lengthy, complex and interactive. There are many ‘boundary-crossing’ moments within it and between it and related or contributory fields. From exploration knowledge through examination knowledge to exploitation knowledge there is, as a science-based industry, an enormous range of diverse knowledge categories and specialities to be encompassed in Life Sciences and biotechnology. That it is a clustered industry almost goes without saying, as wherever it exists as a sector, it congregates around the leading knowledge centres, namely major universities, hospitals and special research institutes. It is an unusual industry in that there is great faith in its capability to deliver miraculous treatments for hitherto incurable diseases like cancer, especially now the Human Genome has been decoded. 4.9 Yet few firms make profits, but they prosper on vast public research budgets and the largesse of big pharmaceuticals firms whose own internal capacities for drug development are under siege. Pharmaceuticals firms are chemistry not biology companies and it is now clear that chemical solvents of multiple diseases that began with penicillin and cortisone are now not likely to re-appear in new guises. So the new ‘molecular biology revolution’ has yet to fulfil its promise while the old paradigm led by synthetic or fine chemistry has had to become biology’s supplicant (Cooke, 2002). A symbol of this new, ‘world turned upside down’ is the DBF, or dedicated biotechnology firm, that has supplanted the large pharmaceuticals firm as 21 the knowledge leader in commercialisation of Life Sciences exploration knowledge. Here are combined many knowledges that hitherto either did not exist or were not so necessary to buy in the market. Building DBFs is a new task of the knowledge economy. Our research shows a wide range of knowledge and its application employed in and around incubators. Bioincubators may, like Hadasit, almost substitute for the market where it does not have the capacity enjoyed in ‘megacentres’ like Boston where MBI has no such demands or problems. 5. Good Bioincubation Management Practices Outside the Bio-Link Targets 5.1 As was noted in 2.1 above, large surveys of bioincubation have commented variably on the practice and policy of bioincubation support. In the US case, they were seen as exerting a positive effect on growth in new technology sectors generally, though this was less clear for biotechnology incubators benchmarked (NBIA, 2002). In a UK report ( CSES, 2003) focused on bioincubation, 22 facilities were examined, were found expensive at £4.6 million average running costs per year but likely to grow in demand for space in the near future. To gain the most up-to-date position, views of incubator managers were solicited on best incubator management practice. An e-mail questionnaire survey was in April 2004 sent to a total of 71 biotechnology incubators across Europe and Israel as entered in the EU Business Incubators Database. The eventual response rate was 10%. Even this low return is useful considering the dearth of research on biotechnology incubator management. CSEL (2001) conducted a wideranging benchmarking study of European incubators which included many biotech incubators. But that study did not include any disaggregated analysis of biotechnology incubators therefore so although the data are referred to here for comparative purposes, the broad scope of the CSEL study means that the validity of the findings for bioincubators is limited. 5.2 As the focus of this report is to examine management good practice, it concentrates on the services and facilities that incubators provide to their clients. The services and facilities can be grouped into the following key functions:- Incubator Space: the provision, management and maintenance of accommodation. The accommodation will usually contain between 10 and 20 incubator units with shared facilities such as common rooms, conference rooms, administrative facilities, scientific and technical facilities, and canteens etc. Business Support Services: pre-incubation services; networking; accounting; entrepreneurship training; financial assistance including advice on raising venture, loans, grants etc; ICT 22 assistance; mentoring; secretarial; legal assistance; IPR assistance; business plan assistance; marketing; training; post incubation services; monitoring and evaluation of performance. While pre-incubation, finance and networking are often provided as a core service, many of these services are provided through external providers. 6. Management Practices That Contribute Most To Successful Performance 6.1 The first of our external respondents is CIDEM-PCB from Spain. As with the French and Canadian examples in the Bio-Link evaluation, the Catalan bioincubator CIDEM-PCB Barcelona indicate three key factors that underlie their good practice. First, they practice being an active strategic partner in their clients’ development. Second, they operate a system where there is continuous coaching and monitoring of the incubated companies. Third, networking is also a key priority: CIDEM-PCB promote interaction with already established companies in their science park, particularly with technological platforms and research groups installed in the park related to specific areas of research. Also like the ‘Latin’ practices in francophone regions, a flexible approach is adopted towards space requirements and development timescales of prospective businesses. 6.2 The second external bioincubator also cites three key elements of their good practice. The bioincubator in question is that of the Babraham Institute at Cambridge, England, Europe’s leading biotechnology cluster in terms of pipeline products. First, Babraham notes the key importance of taking advantage of local and regional research assets in the cluster. This echoes the Barcelona bioincubator’s valuation of networks. Nearby is the Sanger Institute (Genome research), the Laboratory of Molecular Biology (LMB), the Institute of Biotechnology, Cambridge University, Cambridge Science Park and the Cambridge Research Park. 6.3 Second, as well as external cluster connections, Babraham places a strong emphasis on the importance of intra-bioincubator relationships between incubator manager and companies. Because start-ups are so distinctive and different from each other, it is important the bioincubator manager has hybrid social and technological skills. It is clear that company’s needs vary greatly, from requiring a hands-on approach from the incubator manager, others that place demands on an ad hoc basis. Dealing with staff ‘poaching’ by one bioincubator firm from another creates negative social effects. In a real world case this resulted in the affected company becoming fiercely secretive about its operations to the point that they would not involve themselves in any communal incubator activities. While staff poaching is a common 23 practice in business generally, the close knit environment of the business incubator provides a fertile ground for this type of practice. The fostering of good working relationships between start-ups is therefore important to the harmonious functioning of the incubator. Good diplomatic skills are a valuable attribute of incubator managers. The fostering of networking between incubator companies is an important benefit of incubator occupancy that should be encouraged. Because incubator incumbents come from a broad range of backgrounds, from the serial entrepreneur to the first-time academic venture, this places varying demands on the incubator manager and will demand a flexible approach to nurture them through the incubation period. 6.4 Third, the attitude of start-up managers is viewed by some as a key determinant of success. Managers are advised to keep contact with practical realities, there being little place for egoism in business incubators. Where a prospective start-up may believe itself to be the only competitor in the field, it may be devastated to be told that a five minute search on the Internet by an incubator manager had identified dozens of competitors. Thus firm managers require high ‘absorptive capacity’ of the actualité if they are to prosper. It is thus important that bioincubators ensure start-ups form networks and maintain a profile outside the incubator. Attending appropriate conferences and trade fairs is part of a commercial education for startups, helping them make key contacts and raising their profile. 6.5 The three key elements of good bioincubator management communicated by Atlanpole Nantes are the following; networking, entrepreneurship training, and links to big pharma. The development and maintenance by the incubator managers of a comprehensive network of business support services and scientific and technical advisers are key ingredients of a successful incubator. Entrepreneurship training is a key area that has often in France not been provided by the market. Bioincubators collaborate with the education sector to provide courses on entrepreneurship and some run internal courses for their clients. At the Atlanpole, Nantes, managers initiated a degree scheme in entrepreneurship that has been introduced at the University of Nantes. 6.6 From the Atlanpole perspective, successful bioincubators form links with organisations at local, regional and international levels to enhance their knowledge and the quality and range of services they are able to offer their client companies. At local level, incubators form links with private and public sector providers of business support and enterprise, government ministries, financial institutions, technology, science and innovation organisations such as chambers of commerce, education institutions. Third, of prime importance are developing 24 links with big pharma companies who, as Sofinnova reported in 4.3, have for some time tended to sub-contract the early stage research to good quality start-up businesses in their effort to curb R&D costs. 6.7 At IZB Munich, networking was again stressed as primus inter pares of the bioincubator and its incumbent firms’ external tasks. It is surprising how inwardly focused both frequently are. Indeed it seems there is scope for networking training for all concerned, Bio-Link bioincubators and firms included, a conclusion of the Evry meeting in February, 2005. For IZB, typical networking activities include developing links with science, industry and finance. Other links extend to developing relationships with other start-up companies, their graduate companies and politicians. Bioincubators are also members of various scientific bodies including science park associations, government sector initiatives. industry associations and professional bodies. It is vital that firms are engaged in such relationships directly and indirectly. Regionally, too, bioincubators have formal and informal links with many types of organisations such as innovation centres, science parks and research centres, regional development agencies with similar value for firms. 7. Bioincubators and Incumbent Firms Being Regionally Embedded 7.1 Of such importance is embeddedness, and so weakly practised, especially by start-ups, that it is necessary to spell out examples of good practice in more formal dedicated biotech regional network organisations. The Babraham Institute is a member of the Eastern Region Bioscience Initiative (ERBI), an industry-led organisation administered by a Board of Directors and guided by a Steering Group composed of representatives from the biotechnology cluster in Cambridge. It has a membership composed of many of the key organisations in the region including local biotechnology company directors, research institutes, professional service providers and regional development organisations. A core activity of ERBI concerns network meetings on relevant and topical business and scientific subjects, often presented by invited biotech and specialist expert speakers. These meetings are regularly attended by approximately 100 people and provide an important forum for networking with the Cambridge biotechnology cluster. ERBI also fosters networking on an international scale by hosting a major two day European partnering event, the 2005 event follows a large event in 2004 consequent on its successful launch the previous year. Bringing together UK and European biotechnology executives in a dedicated partnering day where delegates met potential business partners informally worked extremely well. Bio-Link found such days among Bio-Link firms to be an eye-opener, surprisingly to the thirty or so firms attending at Evry in 2005. As a further service, ERBI has established special interest 25 biotechnology networks in the areas of purchasing, human resources, finance and business development. The purchasing network offers substantial savings on consumables and office goods, while human resources, finance and business development networks bring together peer groups; sharing expertise and knowledge to enable biotechnologists to operate as efficiently as possible. 7.2 Atlanpole has pursued a formalised approach to its networking activities. It has achieved ISO 9001 certification for each of its three activities: networking and partnership engineering; engineering for innovation; and promotion and corporate investment policy. The ISO 9000 accreditation has become an international reference for quality management requirements in business-to-business dealings. Atlanpole also federates an active group with all local biotechnology firms and participates to different juries including the Ministry of Research, creation and development of new companies, Tremplins Sénat. Less formal but nevertheless useful conduits for knowledge transfer are the ad hoc running of events such as periodic conferences and seminars. A novel networking event in the biotech field has been introduced at the Science Park café of the University of Barcelona called Biotapas, where an invited specialist biotech expert speaks followed by an informal meal of tapas. 7.3 Fostering international networks has many benefits for incubators including learning from incubators management practices in other countries, access to expertise and knowledge perhaps in economies with more mature incubators, scope for collaboration, joint ventures, co-operation or co-incubation of client companies along the lines of Bio-Link and knowledge of biotechnology developments within other companies. International networking is also seen as important and CIDEM-PCB Barcelona (along with Bio-Link’s Genopole) signed formal framework agreements with Quebec Biotechnology Innovation Centre (QBIC). Atlanpole is on the Board of EBN, the European Business Innovation Centre and is on the board of IASP, the International Association of Science Parks. It is linked with biopoles in its twin cities of Cardiff and Seattle. Atlanpole has also recently established formal links for cooperation with the University of Heidelberg. 8. Challenges for Bioincubators 8.1 The location of an incubator within reasonable proximity of key research assets such as university research labs, medical research centres and pharmaceutical firms are key determinants of a successful incubator. One of the most difficult tasks for incubators not located within a cluster is the need to attract start-ups to a non-biotech region. Operating outside a biotechnology cluster, incubators have logistical problems in building strong 26 networks that can provide the outside assistance that start-ups require. Start-ups also find it impossible to benefit from knowledge spillovers through other biotech firms and research institutions. 8.1 Venture capitalists focus their investments on established clusters, often sticking to the one hour drive time from their office rule when considering potential clients. The justification for this seemingly short-sighted attitude is that if there are candidate clients within this catchment, why look further? The opportunity for knowledge spillovers is also restricted in the non-cluster based incubator. The reputation of a region’s research infrastructure has been highlighted by respondents in this study as an important factor in incubator success in a number respects, not least in raising the perception of an incubator to potential investors when start-ups are seeking funding. 8.2 The Babraham Institute encountered challenges working to political timescales rather than sector-specific company development timelines. Biotechnology start-ups have longer gestation periods in business incubators than those in other sectors, hence incubator development strategies are based on growth trajectories commensurate with start-up incubation periods. Pasteur Biotop has an exit policy where clients leave the incubator after two years. Pasteur Biotop has found that while time-limited occupation periods are common, the policy can cause problems where companies are not yet ready or keen to leave the incubator. Lack of space is a common problem for incubators that is often solved by the development of add-on facilities, as with MBI, or completely new facilities. Many incubators only allow start-ups to take space up to a maximum of about 25% of the total incubator space. 8.3 Incubators also frequently point to finance as a challenge, with shortage of national and regional government support, venture capital and lack of in-house seed finance the main problems. CIDEM-PCB noted a lack of other initiatives that might prepare companies for the post incubation period. It is also clear that European incubators adopt policy on firm graduation based on a variety of criteria that determine the timing of their client’s departure from the incubator. The most common criterion applied is to set a time limit on occupation that will usually be in the range 1-5 years with around 3 years being the most popular period. However, many incubators, including Pasteur Biotop, find that tenant companies would like to stay longer than the periods stipulated by incubators. The research and product development process for biotechnology companies is longer than for other sectors, hence incubator policies with time-limited occupancy periods are often based on the need to ensure a 27 constant throughflow of companies that is seen to maintain the dynamism and the constant input of ideas. 8.4 The policy adopted by some incubators of keeping tenants for longer periods is often more to meet the challenge of maintaining an incubator’s income stream rather than being based on sound business and scientific principles. A reasonable strategy is to monitor progress of each start-up on a case-by-case basis and to determine a suitable exit time when the start-up has outgrown or no longer requires the services of the incubator. Some incubators introduce an exit strategy whereby the rent is increased annually during the period of occupation, although some incubators simply raise rents on an ad hoc basis as the need arises to provide an incentive for clients to leave. In line with those reported in other studies, the survey found that occupancy rates of around 80% are most common. Incubator managers however, particularly those running private sector incubators, prefer higher occupancy rates preferably at 100% occupancy to keep the revenue flowing. 8.5 Incubators are increasingly entering into legal agreements with their clients whereby they take an equity stake in, or royalty from, their client companies. A common practice, and one adopted at the Babraham Institute bioincubator, is that if a new client company has no initial finance, the incubator takes a stake of up to a third of the company. Where a company enters the incubator with finance in place, often no equity stake is taken. Those incubators not taking an equity stake or royalties usually adopt an income model based on rental and other sources of income. The issue of direct incubator involvement in client companies through taking equity stakes, raises the question as to whether they are best-placed to judge such decisions. Furthermore, are there conflicts of interest over decisions on incubator operation and company development? 8.6 With many incubators receiving state or regional government support, this assessment and others (see CSES, 2002 for example) find that these sources together with rental income from clients are the main funding streams for incubator operation. Other funding sources include providing business services such as management consultancy and mentoring services to clients. In many state funded incubators, these services are provided in-house and the cost is included in the rent. There is much debate as to whether this incubator model provides the most efficient and cost effective service to clients: clearly not all clients will require all the inhouse services available. However, proponents of this model argue that without these services, the incubator cannot fulfil the criteria of a true incubator facility. Facilities that do not provide a range of in-house business and technical services are viewed as real estate operations such 28 as business parks. Other incubators raise extra income for their incubators by charging their client companies for the provision of technical and administrative services in addition to the rent. 8.7 While some incubators reported marketing activity, few actively prospect for potential incubator entrants. The majority of incubators, particularly those that are in advantageous locations,or have well-established reputations, rely on the steady stream of start-ups approaching them with proposals and business plans. For incubators in more specialist fields, highly focused search activities are conducted e.g. at BioBask in Bilbao, Spain which specialises in proteomics research and incubation, advertisements were successfully placed in Nature Biotechnology to recruit researchers and potential proteomics entrepreneurs. The perceived need was to ensure that the best and most appropriate start-ups are identified, assessed and if successful, recruited. To be effective, the scope of prospecting activity thus embraces networking on a regional, national and international basis. Start-up companies and university spin-offs are the main origins of incubated companies, other client companies arrive from a branch of existing firm. 8.8 The quality of the selection process is highlighted by Pasteur Biotop, which scrutinises the business plans of prospective tenant companies. The entrepreneurial and managerial qualities of the scientist/manager are the only attributes of potential clients that were viewed by all respondents to this survey as very important when selecting clients companies for their incubators, entrepreneur quality being viewed ahead of the quality of the science in considering clients. This finding is however, in concert with findings in North American incubators, where the entrepreneurial capability of potential managers is the key asset they are looking for. 8.9 There is no benchmark period for evaluation of potential clients. For the Babraham Instutute, the evaluation process for start-ups may take 6 weeks although the actual time spent may be just 2 to 3 days. For companies that are pre-funded, the process is less rigorous and this may take just a day. Institute Pasteur report that their period of evaluation varies appreciably by company and while the period may be over many months, the actual time spent on the process itself may be just a few weeks. Babraham Institute has an advisory group of investment advisors to vet potential entrants whose role is to act as ‘champions’ to lead the discussions with inventors. All members of the group are ex-early stage venture capital groups. 29 8.10 The main challenge for companies is raising finance. This underlines the need for incubator managers to focus on this aspect of their responsibilities. The development of good working relationships with financial institutions is crucial in order to provide companies with access to finance for survival and development through the many stages in a company’s progress. The funding problem relates to all types and stages of funding requirements, with incubators reporting lack of seed finance, particularly between €200,000 and €1million second stage and development finance. A particular problem faced by start-ups is lack of expertise in negotiating with venture capital firms, often because companies do not have the necessary financial background. Because the stakes are high, it may be wise for start-ups when negotiating with venture capitalists to employ the services of a financial intermediary to ensure that the companies understand the full implications of the proposition at hand and that the best deal is struck. Start-ups must be conscious of the need to avoid giving away too much equity in their companies for too little investment while on the other hand securing the finance necessary to progress their development. Many incubators even have to help start-ups preparing business plans to present to venture capitalists. 8.11 In many countries and regions, there is a shortage of venture capitalists that specialise in biotechnology start-ups. In Italy, for example, the venture capital market is not that well developed in the life science field and there, difficulties are experienced attracting venture capitalist companies from Europe. Venture capitalists tend to focus their operations where there is a biotechnology cluster and (as mentioned above) this means limiting their activities locally or regionally. Venture capital will tend to look to new markets only where there is a clear opportunity or where their existing markets are saturated. This has been the case for US venture capital which has in recent years entered the German market to expand, however, the German market has experienced a significant downturn in recent years. The investment characteristics of the venture capital industry underline the key need for incubators to develop truly global networks. Some incubators such as the Institut Pasteur are fortunate to have a special link, through shareholder interest, in two specialist biotechnology venture capital companies. The Institut however, report that the process of attracting venture capital is too long in the biotechnology field. At CIDEM-PCB, there have been difficulties recruiting qualified managers to build a balanced managerial team. Furthermore, they have problems accessing market and competition intelligence. 8.12 Many incubators located on university or research institute campuses are in the advantageous position of being able to offer their clients the use of scientific and technical facilities and expertise of these institutes. There are a variety of arrangements under which 30 these facilities are used, ranging from straightforward rental to formal collaborative agreements. Where incubators are a facility of a university, the financial consideration for these arrangements is often at pro bono rates. It is clear that incubators based outside a research institute will have to set up such arrangements and often pay market rate. Location within a research institute/university also has advantages of the expertise of their personnel. Many research institute-based incubators take advantage of the pool of relatively cheap student labour that is available. 8.13 Some few incubators in the survey do not have formal review procedures in place for their start-ups. Incubators without performance review have less formal meetings arranged on an ad hoc basis. This findings is broadly in agreement with the CSES Benchmarking of Business Incubators Report, which found that 34.6% of business incubators do not have such arrangements in place. Bio-Technium in Wales has an annual and quarterly client review procedure provided thorough monitoring. The setting of milestones in conjunction with formal reviews is a useful target for companies to aim for and be assessed at the review meetings. 8.14 The most cited reason for tenants leaving an incubator is lack of space. While incubators, particularly newer purpose built facilities, often have flexible space capable of expansion and contraction as the need arises, the limited space within most incubators means that inevitably companies will need to move out of incubators to expand. Incubator managers are often willing to accommodate company’s space requirements through the takeup of further units, however this practice clearly blocks the entry of new start-ups into the incubator and this may have negative consequences. A further key reason for leaving is that many incubators have a fixed period of occupation, although many incubators are to a degree flexible on this. Companies also cite the availability of cheaper accommodation as a key factor in their decision to leave. Once the unique package of services offered by an incubator is no longer required, there will inevitably be cheaper accommodation available elsewhere to attract startups. 8.15 Finally, in line with research by CSEL (2001) our results suggest the typical business incubator will have on average 2.3 management level staff. On our evidence, the number of incubator managers is two per incubator. The majority of incubator managers in the survey come from the biotechnology industry with most scientist/entrepreneurs. The nature of the business is such that there is fairly high risk of failure for start-ups. More entrepreneurial scientists run many European incubator facilities across Europe. The special skills and the ability to take risk are attributes that are well suited to the incubation management role. Many 31 incubator managers are former biotech company start-ups themselves and therefore understand the peculiar requirements of biotechnology start-ups. 9. Conclusions & Recommendations 9.1 What did we learn in consequence of our in-depth interrogation of eight incubators and their self-perceptions (Weick, 1995)? Three key things. First, in terms of the span of knowledge control, it does not matter whether an incubator is publicly or privately governed. Rather it depends on the regional setting. Thus Hadasit is trust-funded in a thin institutional, but thick exploration knowledge environment. Oxford BiotechNet is different, also trustfunded, but in a thick institutional and research environment. It thus has less gaps to plug. It is closer to BioM although much more sparingly funded. The two public incubators are very different. In Paris there is knowledge but ‘prospecting’ is hard. In Sardinia there is a genetic gold mine but few takers. In North America, the market assists ‘boundary-crossing’ much more than in Europe and Israel but throughput of firms is not markedly higher. Yet, it must be assumed, financing being easier and incubator candidates more numerous, that those passing through bioincubation have a more secure future. 9.2 Second, even so, public determination to create ‘constructed advantage’ bears some fruit and serendipitous investment (e.g. Tiscali) from a separate sector may create a means of exploitation of unique knowledge assets. Where such assets are absent but research infrastructure is strong, incubators are advantageous to extract commercial businesses from the laboratory bench, as in the other cases. However, there may be, as yet untried, better models of commercialisation to explore, even where markets may not be deep and support services abundant. Either this is less difficult, as with Oxford, MBI and Broad Hollow, where selection is done elsewhere in the knowledge value chain, or it is more difficult , as in Genopole or Hadasit where incubators feel they must also perform a rigorous selection function that normal market-facing business start-up business practice looks after in North America. Here, co-incubation is an attractive option 9.3 Finally, ‘boundary crossing’ institutions with networks based on social capital such as bioincubators are extremely important ‘hybrid’ knowledge management intermediaries without which clusters would probably not exist in less accomplished regional settings and have had to be invented even in richer business service environments for biotechnology innovation. Even so, it is noticeable that pure profit incubators are by no means a predominant form taken by incubation in general, notably not in the USA although independence from subsidy is in sight in MBI and an aspiration in QBIC, and none of our sample falls into that 32 category. This kind of knowledge transfer has yet to find its market although it remains essential to the proper functioning of markets. This was particularly pronounced in the responses of the extra European bioincubator managers interviewed in the second bioincubator managers survey. 9.4 Key recommendations on bioincubation as a prelude to co-incubation are thus as follows. Bioincubator managers must build wide and varied institutional and business networks, especially financial networks of supportive investors Firms must undergo training in good search and networking practices Bioincubators may more usefully spend management time on both search and networking activity than over-bureaucratised recruitment measures There is need for strong in-house scientific and technological guidance and good internal bioincubator synergies Bioincubator managers must have ‘hybrid skills’ and if necessary receive training in these Bioincubation is mainly a subsidised activity but sustainability depends on bioincubator fund-raising for greater autonomy. Managers should seek as much institutional autonomy as possible, while recognising few incubators worldwide make significant profits Acknowledgements This research was funded by the European Union DG Enterprise IPS Programme. We are grateful to the sponsor, and to the Bioincubator managers who gave of their time to answer the lengthy interview questions necessitated by the research. None of these is responsible for the interpretations, and acts of commission or omission contained in the article. 33 Best Incubation Tool-Kit – The Co-Incubation Perspective 1. Introduction: One of the objectives of the Bio-Link project was to design a toolkit for Best Incubation Practices (BIP Toolkit) in biotechnology. This toolkit will be based on the experience of the participating incubators as well as that of other leading US incubators, and make recommendations on the best tools to be used in incubation. These tools will refer to main aspects of incubation such as technology transfer from universities, proposal evaluation procedures, financing and holding structures, structuring the companies and business development. The second objective was to validate an international co-incubation scheme that will enable biotechnology start-up companies to benefit from the capabilities and expertise of different incubators. To achieve this goal, an international consortium of five biotechnology incubators, implementing different incubation methods, was formed. Their complementary advantages as well as their experience in different incubation processes will be analyzed and a coincubation scheme will be tested on 10-15 start-ups (2-3 from each participant). The success of the scheme will be assessed using a set of pre-defined impact indicators. Indicators will include scientific achievements, capital formation, strategic partnering, number of employees, contracts, and patenting. The performance of the participating start-ups will be compared with other start-ups that did not participate in the scheme. The third objective was to analyze the extent to which such co-incubation scheme can facilitate the process of start-up creation and development in developing areas. In order to achieve this goal, 2-3 start-up companies from a developing region will participate in the scheme. Their success will be measured against other biotechnology start–up companies in the same region using a set of pre- defined impact indicators. Other objectives of the Bio-Link project referred to analysis of the applicability and transferability of the project to other fields of technology and to other regions such as: - The applicability of the biotechnology BIP-Toolkit - The applicability of the co- incubation model. 2. Findings Supporting Best co-Incubation Practice Tool Kit 2.1 Selection criteria for Best Co-incubation Practice Tool kit The tenant companies belonging to the five participating bio-incubators in the Bio-Link project can be divided into 3 major categories:- 34 a) Companies developing platform technologies (for example: new method for identifying drug targets on cell surface, on-line bioinformatics software solutions)) b) Service-providing companies (for example: analytical services, animal toxicology, clinical trials services) c) Product-developing companies (specific drugs – small and large molecules, medical devices) The selection of a company for a successful co-incubation should follow the following criteria:a) For companies developing platform technology:- Post proof of concept. Has the method been tested in an actual experiment and has it resulted in satisfactory and well documented results?) - Suitable for wide range of collaborations. Can the platform be used by many potential partners without granting exclusivity to any of them? In the case of on-line bio-informatic software this is clearly obvious. b) For service-providing companies: - Meeting a defined need. Does the service meet a real need for a wide range of customers? Is it unique in any way in comparison to existing competitors? - Existing solution. Is the service in place in terms that it can be provided in a short time to potential customers? Or, how long will it take for the company to reach this status? - Certified. Has the company received all the required certificates from regulatory services or other authorities to provide the service (EMEA, EU notified bodies, FDA etc.) c) For product-developing companies (diagnostic kits, medical devices or therapeutic molecules): - Proof of concept (alpha-site, animal model). Was the product tested in an acceptable experimental model whether in animals or in the lab to enable potential partners to evaluate it using consensus scientific tools? - Fast implementation. How long will it take to transfer the technology to a potential coincubation partner? Are standard operating procedures in place? Have they been validated inhouse prior to their being transferred to a potential partner? -Short time to realization. New therapeutics require an extremely long development time. The co-incubation process should take just one aspect of the development – for example the development of an assay in cells to test the efficacy of the molecule. This is a task that can be accomplished within a short period (12-24 months). In addition the following parameters should play a major role in the selection of a company for co-incubation:35 Maturity – companies which are in their seed phase (conception) are not ready for collaboration due to their need to focus and accomplish significant milestones in a short time and under considerable budgetary restraints. Therefore companies which are ready for coincubation should be 2-3 years old. This age will also result in internal clarification and understanding of the needs for co-incubation in terms of the benefits that the company can obtain with such potential collaborations. Critical mass – the need to allocate well-trained scientific and technological staff for the coincubation with a potential partner is impossible for a company with less than 8 employees. Co-incubation requires a dedicated scientist/engineer who can devote most if not all his time to the project. 2.2 Proposed Modes of Best co-Incubation Practice Six potential modes of co-incubation were examined during the Bio-Link project:a) Shared R&D b) Provision of services c) Technology transfer d) Material transfer/sale e) Joint projects/products f) Joint application for R&D funding a) Shared R&D requires defined splitting of the tasks between the parties, lots of exchange of information and travel, dedicated budget, and hence intensive managerial attention – this as we found is an unfavoured mode of collaboration. b) Provision of services. This mode of co-incubation is easy to implement if the serviceproviding company has everything in place. It provides the company with income while it provides the co-incubation partner with a solution to a defined problem(for example, a validation of a difficult to validate Bioanalytical assay). We found this mode the most favoured mode of co-incubation c) Technology transfer/sale. This is also an easy to implement mode of co-incubation. Companies ready for technology transfer are usually prepared to do so in terms of their procedures and certification. For example, this is the case of companies providing bioinformatics on-line software solutions. We found this mode of collaboration favoured by the relevant participating Bio-Link portfolio companies. d) Material transfer/sale. This mode of co-incubation does usually not apply to young companies which do not have ready products. Products under development are usually not 36 shared with other companies due to IP considerations. We found this mode of coincubation unfavoured by the participating portfolio companies. e) Joint projects/products. Young companies do not have the resources to share projects with other companies. These resources refer to financial ones, management attention, legal aspects related to the joint project and lack of personnel in the young companies. For these reasons, this mode of potential co-incubation was rejected by Bio-Link portfolio companies. f) Joint application for R&D funding. This co-incubation mode requires strict definition of the sharing of the IP. Since the IP of young incubator companies is their most valuable asset, this mode of collaboration was not greatly favoured by the portfolio companies. 2.3 Level of interest and motivation for co-incubation We found that the expected level of motivation for co-incubation of young incubator companies is directly related to their field of activity:For companies within the Bio-Link consortium, the level of interest and motivation in coincubation was as follows: - High for service-providing companies - High for start-ups in commercial phase - Moderate for platform technology start-ups - Low for all companies in early development stage As to the motivation for co-incubation of young Bio-Link portfolio companies within an incubator to co-incubate with companies outside the Bio-Link consortium, we found the following:- High for service-providing start-ups - High for start-ups in commercial phase - Moderate for platform technology start-ups - Low for all companies in early development stages 2.4 Key success factors for co-incubation practice Five major success factors were identified as key for co-incubation between young biotech companies in the participating incubators:a) Technology b) Incubator's support c) Company's management d) Budget e) Time 37 a) Technology. A company is ripe for co-incubation if and only if its technology has reached beyond the proof of concept phase. This means that it has been tested successfully in applicable models several times and has been reviewed both internally as well as by outside experts. In addition, the technology must be transferable. This refers to procedures that are well documented in form of scientific papers or in the form of industry style Standard Operating Procedures. b) Incubator's support. Being a complex task, co-incubation requires the involvement of the host bio-incubator. This involvement includes several important factors:- Networking to the other incubator, biotech and pharma companies. - Follow-on of the activity - Administrative support (legal, IP, etc.) - Involvement of experts and consultants to assist in accomplishing the co-incubation objectives. c) Company's management. The management of a start-up company must be devoted to success of the co-incubation. Unless this happens, the driving force of the process is lost. The involvement should be both of the CEO and the R&D manager at a level of the results and the problems encountered during the mutual work. In particular, a manager for the co-incubation project should be appointed. Without a project manager the project is bound to fail! d) Budget. Co-incubation requires an allocated budget. This budget should support materials, labour and overhead and should enable travel between the co-incubation partners. Without a special co-incubation budget, in a company with limited resources, the project will fail since it will take financial resources from the company's ongoing activities. It is recommended that a minimal annual budget of € 50,000 will be spared for co-incubation. e) Time. The basic time unit in the bio-business world is 6 months. Therefore coincubation should be handled with patience. Just the mutual review of the technology may take 6 months and the signing of the agreements another 6 months. Results from a co-incubation project can therefore be expected after 2 years or even 3.The coincubation process is aimed at strengthening the companies’ R&D capabilities to support future business development and it should be viewed within the long timescale which is required to develop a new drug, device or method in the healthcare industry. f) 38 3. The optimal incubation model to support Best Co-Incubation We propose that the optimal bio-incubator should be based on the "all in one" model. Coincubation is dependent on the services and the surrounding business environment provided by the host incubator. The analysis of the 5 participating incubators in Bio-Link (described in a separate part of this report) has clearly demonstrated that portfolio companies belonging to bio-incubators which provide vertical services have better chances for co-incubation than those belonging to incubators that provide only part of these services. The ten "pillars" for the "all in one" models include:a) In-house biotech technological infrastructure b) Access to and collaboration with industry c) Access to business development experts d) Networking e) Access to VCs & the financial community f) Access to service providers (legal, IP, accounting) g) Collaboration with local academic institutes h) In-house science & technology guidance i) Synergy with next door neighbour j) Public relations a) Technological infrastructure. The bio-incubator should be able to provide space and infrastructure for a variety of technologies. This includes upstream and downstream technologies related to products derived form various hosts such as bacteria, yeast and mammalian cells. The incubator should be able to support synthetic chemistry activities. An attractive option is that the incubator should own some of the expensive equipment and allow the tenants to use it. b) Access and collaboration with Industry. The bio-incubator should establish relations and collaborations with the healthcare industry. The projects in the bio-incubator should be presented on a periodic basis to companies in the sector. In addition, the technical support of these companies can be very beneficial (use of equipment, materials animal facilities etc.). It is the responsibility of the bio-incubator manager to ensure that his projects obtain a high level of exposure to the healthcare industry. Bio-incubators with strong relationships with the industry have a clear advantage in promoting co-incubation and business development as compared to those which do not give enough emphasis to relationships with industry. c) Access to business development experts. Each bio-incubator should have a director of business development whose responsibilities will include partnering, preparation of 39 presentations, deal structuring and analysis assistance in the preparation of business plans. The lack of this function in most of the partnering bio-incubators in Bio-Link was the main reason for the lack of co-incubation deals in the first year of the project. The function of business development can also be split between several experts in different fields. It should be remembered that most, if not all, of the entrepreneurs are scientists that lack the experience and the culture of the healthcare industry and thus tend to make many mistakes during the early years of their companies. d) Networking. This is a joint task of all the members of the management of the bio-incubator. Networking can be split into personal networks which are the result of the relationships created during the years between the members of the bio-incubator to external organizations, industry, academia, the financial community etc. Another form of networking is the virtual one which is based on databases provided on the internet. Access to literature and reports issued by expert companies are part of this virtual networking. We found out that some of the incubators are unfamiliar with the current publications related to bio-business such as BioWorld Today etc. Networking was found to be the major parameter appreciated by tenant companies in all the five partner incubators of the Bio-Link project. e) Access to VCs and financial community. This is clearly a key factor for the success of each entrepreneurial organization. Connections to the financial community refer to angels and private investors, local banks, global VCs, local VCs and also to the healthcare industry. A basic business model is an essential tool for each company in a bio-incubator to enable it to present its business concept to these potential investors. The incubator should arrange periodic meetings with potential investors and be able to sell the 3 basics of a bio-start-up: Technology, Networking and Dream. f) Access to service providers. The bio-incubator should be able to take care of all the administrative tasks which face a young tenant company . These includeaccounting and bookkeeping, legal services, intellectual property services and personnel recruitment agencies. Provision of these services to the tenant companies should enable the CEOs of the young entrepreneurial companies to focus on their technology objectives. g) Collaboration with local academic institutes. The relations with academia are an essential service that should be provided by the bio-incubator. Such relations contribute to the flux of projects that are forwarded from the bio-incubator. They also are essential as a source of scientific experts who can serve as consultants and as members of the Scientific Advisory Boards of the tenant companies. A relationship with a prestigious academic institute is also a key success factor in fundraising and for the public relations of the bio-incubator. The relations between the bio-incubator and the academic institute should be almost symbiotic such that the bio-incubator is the default address to which young entrepreneurs from the 40 university are referred by their Technology Transfer Offices. On the other hand, the university should be able to present the bio-incubator as its window for the "applicative world". h) In-house science and technology guidance. This guidance refers mainly to technological aspects which are part of the development process in the healthcare industry. Pharmaceutical development, clinical development and regulatory affairs are just part of the guidance required by young biotech and pharma companies. Guidance in these fields, which usually are part of the more advanced stages of a project, are essential from day zero since they have a cardinal impact on the company's development plans, business model, likelihood of registration and hence on fundraising. i) Synergy with next door neighbour. Bio-incubators should try to form an internal technological cluster. This recommendation refers mainly to basic technologies such as genetic engineering, monoclonal antibodies etc. Internal clustering can contribute to real sharing of expertise and resources. However, its mainly advantage is in business development and accumulation of internal expertise in a certain field. Companies with similar technologies can use the same basic development equipment which is usually quite expensive and cannot be justified for one company, especially if it is not routinely being used. j) Public relations. Young biotech companies are "selling dreams". It is within this scope that the bio-incubator must provide them with an optimal exposure to the printed and electronic press. Public relations have a major influence on the financial community, especially on fundraising which is so essential for any young company. Therefore the bio-incubator should hire the services of a professional public relation company as part of its service package to its tenant companies. 4. Additional observations and recommendations for an efficient BIP tool-kit. 4.1 Business development. Co-incubation is primarily aimed at business development. Since all the participating incubators lack a position of "incubator business development manager", it is strongly recommended that for each co-incubation imitative, an accompanying expert in business development should be appointed. This refers mainly to the 3 major professional disciplines: drugs, medical devices and platform technologies. The responsibilities of the business development advisor will include the review of the business impact of the co-incubation in terms of its relative contributions to both parties. A business development "mentor" can also explain and clarify the benefits of the co-incubation to the management of young start-up companies, which in most cases lack the necessary business experience. Once an agreement or a term sheet is in the process of being issued, his role will be to review it and adjust it to the 41 actual needs of both partners. In most cases, this assistance refers to simplifying the agreement. 4.2 Consolidation. Bio-incubators should try to consolidate their co-incubation efforts. This refers mainly to their portfolio companies in a specific field, for example, new drugs for cancer. In this case, the desired co-incubation model will aim at one major partner collaborating with all the portfolio companies dealing with cancer in that specific incubator. This model has several advantages as compared to the one-on-one model. In the first place, it reduces the risk for the partnering company. Then, it enables the interested biotech or pharma company to save time and resources by reviewing several companies in "one glance". Such collaboration creates a lot of motivation and drive for the incubator tenant companies and will also enable the incubator to attract better new companies which are active in a specific field and create a selection between the candidate companies. 42 5. Summary Best co-Incubation Practice Tool Kit Optimal incubation model Selection criteria for coincubation Key Success Factors for Coincubation ‘All in one’ model: - In house biotech technological infrastructure - Access to and collaboration with industry - Access to business development experts - Networking - Access to VCs & financial community - Access to service providers (legal, IP, accounting) - Collaboration with local academic institutes - In-house science & technology guidance - Synergy with next door neighbour - Public relations a)Technology developer Wide scope Ready for implementation and/or technology transfer b)Service provider Certification Technological background material + website Fast response Accessibility c) Maturity Post “creation hassle” – 2-3 years old d) Critical mass – not less than 8 employees 1. Technology Beyond proof-of-concept “Transferable” – at “operating procedure” level 2. Incubator Co-incubation oriented and supportive Business development guidance – from day zero Administrative assistance (TT and MTA template documents in place) 3. Company’s Management Willingness and openness to collate and share (language & culture gap) Managerial awareness and attention Co-incubation project manager 4. Budget Financial resources allocated for co-incubation – labour, material, travel 5. Time A successful co-incubation requires at least 2-3 years from conception. Likelihood of co-incubation success High for service-providing companies Medium for platform technology companies Low for developers of molecules High for companies in commercial stage Medium for companies in transition (pre-commercial) 43 Low for early stage companies References Consulted Andersson, M. & Ejermo, O. (2002) Knowledge production in Swedish functional regions 1993-1999, paper to International Workshop on ‘ Knowledge Spillovers and Knowledge Management in Industrial Clusters and Networks’, Ljungby, Sweden, 1921 September (published in C. Karlsson et al. (eds.) (2004) Knowledge Spillovers & Knowledge Management, Cheltenham, Edward Elgar) Audretsch, D. & Feldman, M. (1996) Knowledge spillovers and the geography of innovation and production, American Economic Review, 86, 630-640 Audretsch, D. (2001) R&D spillovers and the geography of innovation and production, paper to International Workshop ‘Innovation Clusters and Interregional Competition’ Kiel Institute of World Economics, November 12-13 (published in J. Bröcker, D. Dohse & R. Soltwedel (eds.) (2003) Innovation Clusters & Interregional Competition, Berlin, Springer) Centre for Strategy & Evaluation Services (2003) Provision of Bioscience Incubators and Grow-on Facilities in the UK, London, CSES Cooke, P. (2001) Regional innovation systems, clusters, and the knowledge economy, Industrial & Corporate Change, 10, 945-973 Cooke, P. (2002a) Knowledge Economies, London, Routledge Cooke, P. (2002b) Regional science policy and the growth of knowledge megacentres in bioscience clusters. Presented at the Regional Science Association 42nd European Congress, Dortmund, Germany, August 27-31, 2002 (published in Special Issue – Embeddedness & Regional Development: Guest Editors, Frans Boekema & Roel Rutten, European Planning Studies,12, 2004) Cooke, P. (2004) Rational drug design, the knowledge value chain and bioscience megacentres, Cambridge Journal of Economics, 28, (forthcoming) Cooke, P, Roper, S. & Wylie, P. (2003) ‘The ‘golden thread of innovation’ and Northern Ireland’s evolving regional innovation system, Regional Studies, 37, June Feldman, M. & Audretsch, D. (1999) Innovation in cities: science-based diversity, specialisation and localised competition, European Economic Review, 43, 409-429 Fritsch, M. (2001) How and why does efficiency of Regional Innovation Systems matter? Paper to International Workshop on ‘Innovation Clusters & Interregional Competition’, Kiel Institute of World Economics, Kiel, Germany, November 12-13 (published in J. Bröcker, D. Dohse & R. Soltwedel (eds.) (2003) Innovation Clusters & Interregional Competition, Berlin, Springer) Giesecke, S. (2000) The contrasting roles of government in the development of biotechnology industry in the US and Germany, Research Policy, 29, 205-223 Granovetter, M. et al (2000) Social networks in Silicon Valley, in Lee, C. et al. (eds.) The Silicon Valley Edge, Stanford University Press Jaffe, A, Trajtenberg, M. & Henderson, R. (1993) Geographic localization of knowledge spillovers as evidenced by patent citations, Quarterly Journal of Economics, 108, 577-598 Kaiser, R. (2003) Multi-level science policy and regional innovation: the case of the Munich cluster for pharmaceutical biotechnology, European Planning Studies, 11, 841-858 National Business Incubation Association (2002) A National Benchmarking Analysis of Technology Business Incubator Performance & Practices, Washington, NBIA Norton, R. (2000) Creating the New Economy: the Entrepreneur and the US Resurgence, Cheltenham, Edward Elgar 44 Owen-Smith, J, Riccaboni, M, Pammolli, F. & Powell, W. (2001) A comparison of US and European university-industry relations in life sciences, (mimeo) Porter, M. (1998) On Competition, Boston, Harvard Business School Press Powell, W, Koput, K, Bowie, J. & Smith-Doerr, L. (2002) The spatial clustering of science and capital: accounting for biotech firm venture capital relationships, Regional Studies, 36, 291-305 Senker, J. & Van Zwanenberg, P. (2001) European Biotechnology Innovation Systems, Final Report to EU-TSER Programme, University of Sussex, SPRU Weick, K. (1995) Sensemaking in Organizations, London, Sage Wolter, K, (2002) Can the US experience be repeated? The evolution of biotechnology in three European regions, (mimeo), Duisburg University, Germany Zook, M. (2000) Grounded capital: venture capital’s role in the clustering of Internet firms in the US, paper to American Collegiate Schools of Planning Conference, Atlanta, November Zucker, L, Darby, M. & Armstrong, J. (1998) Geographically localised knowledge: spillovers or markets? Economic Inquiry, 36, 65-86 45