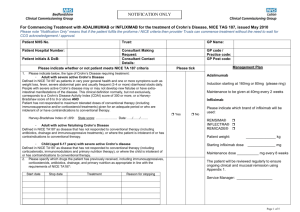
Remicade CC
advertisement

Utilization Review Policy Subject: infliximab for IV injection (Remicade®) Date revised: 12/09/2009, selected revision 01/13/2010 Description Infliximab is a tumor necrosis factor (TNF) antagonist that is approved by the Food and Drug Administration (FDA) for the following indications. Rheumatoid arthritis in combination with methotrexate Psoriatic arthritis Ankylosing spondylitis Plaque psoriasis Crohn’s disease or fistulizing Crohn’s disease Ulcerative colitis Remicade is available as a lyophilized powder and is given by IV infusion over at least 2 hours. It is available as 100 mg of infliximab in a 20 mL vial. After reconstitution, the product must be further diluted to 250 mL with 0.9% sodium chloride injection. Premedication with antihistamines, acetaminophen, or corticosteroids may be required to prevent adverse effects during the infusion. Indications, Medically Necessary 1. RA in adults: Indicated for the treatment of moderate to severe active RA in patients who meet all of the following criteria. Infliximab is prescribed by a rheumatologist, and Patient has had an inadequate response to at least 2 months of therapy with one of the following non-biologic DMARDs: methotrexate, hydroxychloroquine, leflunomide ® (Arava ), or sulfasalazine, and Exceptions to having tried a non-biologic DMARD for 2 months can be made in the following circumstances: The patient could not tolerate the non-biologic DMARDs or if there are contraindications to all four of these agents OR The patient has recent onset RA within the previous 6 months AND infliximab will be given in combination with methotrexate unless there is a contraindication to methotrexate AND the patient has high disease activity as determined by the physician (document physician’s method for determining disease activity) or has a poor prognosis. Markers of poor prognosis include functional limitations based on the Health Assessment Questionnaire (HAQ) score or other functional status measures; extraarticular manifestations of the disease (e.g., rheumatoid nodules, RA vasculitis, secondary Sjögren’s syndrome, Felty’s syndrome, RA lung disease); positive 12/09/2009 1 Infliximab (Remicade) rheumatoid factor (RF) and/or positive anti-citrullinated peptide (CCP) antibodies; and/or bone/joint erosions by radiography. Patients with early RA and low or moderate disease activity are usually not started on infliximab or another biologic DMARD as the initial therapy. Note: these patients should have tried a self-administered TNF antagonist before infliximab. See next criteria. Patient has tried a self-administered TNF antagonist, adalimumab (Humira), certolizumab pegol (Cimzia), etanercept (Enbrel), or golimumab (Simponi) for at least 2 months and had an inadequate response or was intolerant to one of these other TNF antagonists, and Infliximab will be used in combination with methotrexate. Infliximab is usually given in combination with methotrexate, but it has also been used in combination with leflunomide or other oral DMARDs in patient who have contraindications to or intolerant to methotrexate. Infliximab has also been used as monotherapy for RA. For patients who are continuing with infliximab there must be documentation that the patient is or is not on another DMARD in conjunction with infliximab and the other drug therapies that have been used to treat RA. Dosing in RA: 3 mg/kg IV infusion followed by additional similar doses at 2 and 6 weeks after the first infusion, then every 8 weeks thereafter. Should be given in combination with MTX if possible. If the patient has an inadequate response to 3 mg/kg, the dose can be increased gradually up to a maximum of 10 mg/kg. In patients who lose response before the next dose is scheduled, the interval can be decreased to every 7, 6, 5, or 4 weeks or the dose per infusion may be increased. The maximum dose is 10 mg/kg and the shortest interval is every 4 weeks. Background: Many studies have shown that most patients with RA who respond to infliximab will respond to the 3 mg/kg every 8 weeks dosage. Increasing the dose and/or reducing the interval between infusions may increase the percentage of patients who will respond. Initial approval/extended approval: Initial approval is for 2 months of therapy. This is 3 mg/kg initially and then 2 and 6 weeks after the initial dose. Patients are evaluated for response after the third dose. Approve for an additional 12 months of therapy if the patient has responded (less joint pain, morning stiffness, or fatigue; improved function or activities of daily living; decreased soft tissue swelling in joints or tendon sheaths; improved laboratory values; reduced dosage of corticosteroids) as determined by the prescribing rheumatologist. The patient may not have a full response by 2 months, but there should be some response. If the response is inadequate after 4 doses, the dose can be increased gradually up to 10 mg/kg or a lesser dose can be given at a more frequent interval. The dose may need to be increased in patients whose disease flares after an initial response indicating a loss of response to the drug. 12/09/2009 2 Infliximab (Remicade) Duration of therapy in RA: indefinite if the patient is responding. Intermittent therapy has been used but it is well accepted that therapy should be continuous in patients who respond. Labs/Diagnostics required: Patient should be screened for latent tuberculosis (TB) infection prior to starting therapy and then every year while on infliximab. Screening may include any of the following: history, tuberculin skin testing, interferon gamma release assay (IGRA), chest x-ray. In general, patients with latent TB infection should begin preventive therapy before starting infliximab. Waste management: There is no overfill in the infliximab vials. The initial dose in RA is 3 mg/kg. For patients who are already on infliximab, the dose should be calculated and the number of vials needed assessed. Usually the dose should be rounded to the next whole number of vials to avoid wasting unused medication left in the vial. Dose 3 mg/kg Body weight less than 70 kg Body weight 70 to 100 kg Dose 5 mg/kg Body weight 61 kg or less Body weight 62 to 81 kg Body weight 82 to 100 kg Dose 6 mg/kg Body weight 50 kg or less Body weight 51 to 67 kg Body weight 68 to 84 kg Body weight 85 to 100 kg Dose 7.5 mg/kg 54 kg or less 55 to 67 kg 68 to 80 kg 81 to 94 kg 95 to 108 kg Dose 10 mg/kg 50 kg or less 52 to 60 kg 61 to 71 kg 72 to 81 kg 82 to 91 kg 92 to 100 kg Number of vials 2 3 3 4 5 3 4 5 6 4 5 6 7 8 5 6 7 8 9 10 Exclusions: same for all indications. See below. 2. Psoriatic arthritis in adults: Indicated for moderately to severely active psoriatic arthritis in patients who meet all of the following criteria. Infliximab is prescribed by a rheumatologist or by a dermatologist in consultation with a rheumatologist and 12/09/2009 3 Infliximab (Remicade) Patients with peripheral arthritis, as the principle manifestation of psoriatic arthritis, have had an inadequate response to at least 2 months of therapy with one of the following nonbiologic DMARDs or were intolerant to one of these agents: cyclosporine, leflunomide, sulfasalazine, or methotrexate. Exceptions to trying a non-biologic DMARD can be made for patients with peripheral arthritis who have a poor prognosis as determined by the prescribing rheumatologist or dermatologist. Factors associated with poor prognosis in psoriatic arthritis include the number of actively inflamed joints (polyarticular disease rather than monoarticular disease); elevated erythrocyte sedimentation rate; failure of other medications; presence of damage on x-ray or clinically; loss of function; and diminished quality of life. OR Patients with moderate or severe axial manifestations of psoriatic arthritis should have had an inadequate response to therapy with 2 different oral nonsteroidal anti-inflammatory drugs (NSAID) that have been tried in about 1 month (for example, try one NSAID for 10 to 14 days and try a second for 10 to 14 days). Some examples of NSAIDs are naproxen, diclofenac, ibuprofen, ketoprofen, oxaprozin, piroxicam. OR Patients with psoriatic arthritis with manifestations as enthesitis or dactylitis should have tried a NSAID or a non-biologic DMARD (cyclosporine, leflunomide, sulfasalazine, or methotrexate) for at least 2 months and had an inadequate response, AND Patient has tried a self-administered TNF antagonist, adalimumab (Humira), etanercept (Enbrel), or golimumab (Simponi) for at least 2 months and had an inadequate response or was intolerant to one of these other TNF antagonists. For patients who are continuing with infliximab there must be documentation of the other drug therapies that the patient has used to treat psoriatic arthritis. Dosing in psoriatic arthritis: 5 mg/kg followed with additional similar doses at 2 and 6 weeks after the first infusion and then every 8 weeks thereafter. Other dosing regimens are not FDA-approved for psoriatic arthritis. However, if the patient has an inadequate response to 5 mg/kg, the dose can be increased gradually to a maximum of 10 mg/kg. In patients who lose response before the next dose is scheduled, the interval can be decreased to every 7, 6, 5, or 4 weeks. The maximum dose is 10 mg/kg and the shortest interval is every 4 weeks. Initial approval/extended approval: Initial approval is for 2 months of therapy. This is 5 mg/kg initially and then 2 and 6 weeks after the initial dose. Patients are evaluated for response after the third dose. Approve for an additional 12 months of therapy if the patient has responded (less joint pain, morning stiffness, or fatigue; improved function or activities of daily living; decreased soft tissue swelling in joints or tendon sheaths; improvements in acute phase reactants [for example C-reactive protein]) as determined by the prescribing rheumatologist or dermatologist. The patient may not have a full response by 2 months, but there should be some response. If the response is inadequate after 4 doses, the dose can be 12/09/2009 4 Infliximab (Remicade) increased up to 10 mg/kg or a lesser dose can be given at a shorter interval. Usually the interval between doses is decreased and/or the dose is increased in increments up to 10 mg/kg. The dose may need to be increased in patient whose disease flares after an initial response. Duration of therapy in psoriatic arthritis: indefinite if the patient is responding. It is well accepted that therapy should be continuous in patients who respond. Labs/Diagnostics required: Patient should be screened for latent tuberculosis (TB) infection prior to starting therapy and then every year while on infliximab. Screening may include any of the following: history, tuberculin skin testing, interferon gamma release assay (IGRA), chest x-ray. In general, patients with latent TB infection should begin preventive therapy before starting infliximab. Waste management: There is no overfill in the infliximab vials. For patients who have already been started on infliximab, the dose should be calculated and the number of vials needed assessed. Usually the dose should be rounded to the next whole number of vials to avoid wasting unused medication left in the vial. Dose 5 mg/kg Body weight 61 kg or less Body weight 62 to 81 kg Body weight 82 to 100 kg Number of vials 3 4 5 Exclusions: same for all indications. See below. 3. Ankylosing spondylitis in adults: Indicated in patients with active ankylosing spondylitis who meet all of the following criteria: Infliximab is prescribed by a rheumatologist, and Patient has had an inadequate response to at least 2 months of therapy with one non-biologic DMARD such as sulfasalazine or methotrexate (see DMARD table) or was intolerant to one of these agents, and Exceptions can be made for patients with only axial disease. These patients should have had an inadequate response to therapy with 2 different oral nonsteroidal antiinflammatory drugs (NSAID) that have been tried in about 1 month (for example, try one NSAID for 10 to 14 days and try a second for 10 to 14 days). Some examples of NSAIDs are naproxen, diclofenac, ibuprofen, ketoprofen, oxaprozin, piroxicam. Patient has tried a self-administered TNF antagonist, adalimumab (Humira), etanercept (Enbrel), or golimumab (Simponi) and had an inadequate response after at least 2 months of therapy or was intolerant to one of these other TNF antagonists. For patients who are continuing with infliximab there must be documentation of the other drug therapies that the patient has used to treat ankylosing spondylitis. 12/09/2009 5 Infliximab (Remicade) Dosing in ankylosing spondylitis: 5 mg/kg IV infusion followed by additional similar doses at 2 and 6 weeks after the first infusion, and then every 6 weeks thereafter. Other dosing regimens are not FDA-approved for ankylosing spondylitis. However, if the patient has an inadequate response to 5 mg/kg, the dose can be increased to a maximum of 10 mg/kg. In patients who lose response before the next dose is scheduled, the interval can be decreased to every 5 or 4 weeks. The maximum dose is 10 mg/kg and the shortest interval is every 4 weeks. Initial approval/extended approval: Initial approval is for 2 months of therapy. This is 5 mg/kg initially and then 2 and 6 weeks after the initial dose. Patients are evaluated for response after the third dose. Approve for an additional 12 months of therapy if the patient has responded (less joint pain, morning stiffness, or fatigue; improved mobility, e.g., improved spinal mobility; decreased soft tissue swelling in joints or tendon sheaths; improvements in acute phase reactants (ESR, CRP) as determined by the prescribing rheumatologist. The patient may not have a full response by 2 months, but there should be some response. If the response is inadequate after 4 doses, the dose can be increased up to 10 mg/kg or a lesser dose can be given every 5 or 4 weeks. Usually the interval between doses is decreased and/or the dose is increased in increments up to 10 mg/kg. The dose may need to be increased in patient whose disease flares after an initial response. Duration of therapy in ankylosing spondylitis: indefinite if the patient is responding. It is well accepted that therapy should be continuous in patients who respond. Labs/Diagnostics required: Patient should be screened for latent tuberculosis (TB) infection prior to starting therapy and then every year while on infliximab. Screening may include any of the following: history, tuberculin skin testing, interferon gamma release assay (IGRA), chest x-ray. In general, patients with latent TB infection should begin preventive therapy before starting infliximab. 12/09/2009 6 Infliximab (Remicade) Waste management: There is no overfill in the infliximab vials. For patients who have already been started on infliximab, the dose should be calculated and the number of vials needed assessed. Usually the dose should be rounded to the next whole number of vials to avoid wasting unused medication left in the vial. Dose 5 mg/kg Body weight 61 kg or less Body weight 62 to 81 kg Body weight 82 to 100 kg Number of vials 3 4 5 Exclusions: same for all indications. See below. 4. Plaque psoriasis in adults: Indicated for the treatment of severe chronic plaque psoriasis in patients who meet all of the following criteria. Infliximab is prescribed by a dermatologist, and Patient has a minimum body surface area (BSA) involvement with plaque psoriasis of at least 5%, and Exceptions to having a minimum BSA involvement can be made in the following circumstances: 1) Patients with plaque psoriasis of the palms, soles, head and neck, nails, intertriginous areas (inverse psoriasis), or genitalia are not required to have a minimum BSA involvement OR 2) Patients has had an inadequate response to a 3-month trial of either topical therapy OR localized phototherapy AND an inadequate response to a 3-month trial of a systemic therapy [methotrexate, acitretin (Soriatane), or cyclosporine] AND has significant disability or impairment in physical or mental functioning, according to the treating physician. Note: These patients are not required to meet the criteria in the next bullet point. Patient has tried systemic therapy with methotrexate, acitretin (Soriatane), or cyclosporine or phototherapy with UVB or oral methoxsalen plus UVA light [PUVA]) for psoriasis for at least 3 month, and Exceptions can be made for patients who have contraindications to all of these therapies and if phototherapy is not available. Patient has tried a self-administered TNF antagonist, adalimumab (Humira) or etanercept (Enbrel), for plaque psoriasis for at least 2 months and had an inadequate response or was intolerant to one of these other TNF antagonists. For patients who are continuing with infliximab there must be documentation of the other drug therapies that the patient has used to treat plaque psoriasis. 12/09/2009 7 Infliximab (Remicade) Dosing in plaque psoriasis in adults: 5 mg/kg followed with additional similar doses at 2 and 6 weeks after the first infusion and then every 8 weeks thereafter. Other dosing regimens are not FDA-approved. However, if the patient has an inadequate response to 5 mg/kg, the dose can be increased to a maximum of 10 mg/kg. In patients who lose response before the next dose is scheduled, the interval can be decreased to every 6, 5, or 4 weeks. The maximum dose is 10 mg/kg and the shortest interval is every 4 weeks. Initial approval/extended approval: Initial approval is for 2 months of therapy. This is 5 mg/kg initially and then 2 and 6 weeks after the initial dose. Patients are evaluated for response after the third dose. Approve for an additional 12 months of therapy if the patient has responded (for example, there is less induration, erythema, scaling, itching) as determined by the prescribing dermatologist. The patient may not have a full response by 2 months, but there should be some response. In clinical trials, patients were significantly improved by week 10 of therapy. If the response is inadequate after 4 doses, the dose can be increased up to 10 mg/kg and/or the interval decreased to every 6, 5, or 4 weeks. Duration of therapy in plaque psoriasis: indefinite. It is well accepted that therapy should be continuous in patients who respond. Labs/Diagnostics required: Patient should be screened for latent tuberculosis (TB) infection prior to starting therapy and then every year while on infliximab. Screening may include any of the following: history, tuberculin skin testing, interferon gamma release assay (IGRA), chest x-ray. In general, patients with latent TB infection should begin preventive therapy before starting infliximab. Waste management: There is no overfill in the infliximab vials. The initial dose in plaque psoriasis is 5 mg/kg. For patients who are already on infliximab, the dose should be calculated and the number of vials needed assessed. Usually the dose should be rounded to the next whole number of vials to avoid wasting unused medication left in the vial. Dose 5 mg/kg Body weight 61 kg or less Body weight 62 to 81 kg Body weight 82 to 100 kg Number of vials 3 4 5 Exclusions: same for all indications. See below. 12/09/2009 8 Infliximab (Remicade) Crohn’s disease is divided into induction of remission, maintenance of remission, fistulizing Crohn’s disease and to prevent recurrence of Crohn’s disease after ileocolonic resection. 5A. Crohn’s disease in adults and children aged 6 years and older (to induce remission): Indicated in moderate to severe active Crohn’s disease in patients who meet all of the following criteria. Infliximab is prescribed by a gastroenterologist, and Patient has had an inadequate response to treatment with corticosteroids (systemic), azathioprine, 6-mercaptopurine, or methotrexate and Exceptions to having an inadequate response to these other treatments can be made in one of the following circumstances: Patient has been recently hospitalized for Crohn’s disease and was started on infliximab in the hospital OR Patient has been started on azathioprine, 6-mercaptopurine or methotrexate and a more rapid response is needed than can be provided by these other drugs OR Note: these patients are still required to try a self-administered TNF antagonist, see next criteria. If steroids are contraindicated or not desired, then azathioprine, 6-mercaptopurine, or methotrexate should be tried if they are not contraindicated. Patient has tried a self-administered TNF antagonist, adalimumab (Humira) or certolizumab pegol (Cimzia) for at least 2 months and had an inadequate response or was intolerant to the other TNF antagonist. Exceptions to trying a self-administered TNF antagonist can be made in the following circumstances: Patients who were recently hospitalized for Crohn’s disease and started infliximab in the hospital OR Children or adolescents less than 18 years of age. Adalimumab and certolizumab pegol are not FDA approved in children or adolescents with Crohn’s disease. See criteria below for Dosing, Initial Approval/Extended Approval, Duration of Therapy, Lab/Diagnostics Required, Waste Management, and Exclusions. 5B. Crohn’s disease in adults and children (to maintain remission): Indicated in moderate to severe active Crohn’s disease for maintenance of remission in patients who meet the following criteria. Infliximab is prescribed by a gastroenterologist and Patient has had induction therapy with infliximab and has responded with a lessening in severity of signs and symptoms. 12/09/2009 9 Infliximab (Remicade) See criteria below for Dosing, Initial Approval/Extended Approval, Duration of Therapy, Lab/Diagnostics Required, Waste Management, and Exclusions. 5C. Fistulizing Crohn’s disease in adults: Indicated to reduce the number of draining enterocutaneous (perianal or abdominal) and rectovaginal fistulas and maintaining fistula closure in patients with Crohn’s disease who meet the following criteria. Infliximab is prescribed by a gastroenterologist, and Patient has enterocutaneous (perianal or abdominal) and/or rectovaginal fistulas, and Patient has not responded to conventional treatment such as antibiotics (metronidazole [Flagyl], ciprofloxacin [Cipro]), surgical drainage with examination under anesthesia, and/or immunosuppressive therapy (azathioprine, 6-mercaptopurine, IV cyclosporine). See criteria below for Dosing, Initial Approval/Extended Approval, Duration of Therapy, Lab/Diagnostics Required, Waste Management, and Exclusions. 5D. Crohn’s disease in adults after ileocolonic resections: Indicated to reduce the chance of recurrence after surgery in patients with Crohn’s disease who meet the following criteria. Infliximab is prescribed by a gastroenterologist. See criteria below for Dosing, Initial Approval/Extended Approval, Duration of Therapy, Lab/Diagnostics Required, Waste Management, and Exclusions. Dosing in active Crohn’s disease (5A,5B) or in fistulizing Crohn’s disease in adults (5C): 5 mg/kg as an induction regimen at 0, 2, and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter. If the patient has an inadequate response to 5 mg/kg, the dose can be increased gradually up to a maximum of 10 mg/kg. In patients who lose response before the next dose is scheduled, the interval can be decreased to every 7, 6, 5, or 4 weeks or the dose per infusion can be increased. The maximum dose is 10 mg/kg and the shortest interval is every 4 weeks. Patients who do not respond by week 14 are unlikely to respond with continued dosing and consideration should be given to discontinuing. Dosing in active Crohn’s disease in children (5A,5B): 5 mg/kg given as an IV induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks. If the patient has an inadequate response to 5 mg/kg, the dose can be increased gradually up to a maximum of 10 mg/kg. In patients who lose response before the next dose is scheduled, the interval can be decreased to every 7, 6, 5, or 4 weeks or the dose per infusion can be increased. The maximum dose is 10 mg/kg and the shortest interval is every 4 weeks. Patients who do not respond by week 14 are unlikely to respond with continued dosing and consideration should be given to discontinuing. 12/09/2009 10 Infliximab (Remicade) Dosing in Crohn’s disease in adults after ileocolonic resections (5D): 5 mg/kg as an induction regimen at 0, 2, and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter. Initial approval/extended approval: For inducing remission in active Crohn’s disease in adults and children and in adults with fistulizing Crohn’s disease, initial approval is for 3 doses of infliximab given at weeks 0, 2 and 6. Patients are evaluated for response after the first 3 doses, about week 14, and for further maintenance therapy. If patients do not respond to the 3 infusions, further treatment with infliximab is not recommended. Approve for an additional 12 months of therapy if the patient has responded as determined by the prescribing gastroenterologist. The patient may not have a full response after 3 doses, but there should be some response. If the response is inadequate the dose can be gradually increased up to 10 mg/kg or a lesser dose can be given at a more frequent interval. For patients who respond and then lose response, the dose may be increased to 10 mg/kg every 8 weeks or 5 mg/kg can be given every 6, 5, or 4 weeks. The maximum dose is 10 mg/kg and the shortest interval is every 4 hours. To prevent recurrence of Crohn’s disease in adults after ileocolonic resections, approve for 12 months of therapy. Duration of therapy in Crohn’s disease or fistulizing Crohn’s disease or after ileocolonic resection: indefinite if the patient is responding. It is well accepted that maintenance therapy is indicated if there is a response to induction therapy. Labs/Diagnostics required: Patient should be screened for latent tuberculosis (TB) infection prior to starting therapy and then every year while on infliximab. Screening may include any of the following: history, tuberculin skin testing, interferon gamma release assay (IGRA), chest x-ray. In general, patients with latent TB infection should begin preventive therapy before starting infliximab. Waste management: There is no overfill in the infliximab vials. For patients who have already been started on infliximab, the dose should be calculated and the number of vials needed assessed. Usually the dose should be rounded to the next whole number of vials to avoid wasting unused medication left in the vial. Dose 5 mg/kg Body weight 61 kg or less Body weight 62 to 81 kg Body weight 82 to 100 kg Number of vials 3 4 5 Exclusions: same for all indications. See below. 6. Ulcerative colitis in adults: Indicated in moderately to severely active ulcerative colitis in adult patients who meet all of the following criteria. Infliximab is prescribed by a gastroenterologist, and 12/09/2009 11 Infliximab (Remicade) Patient has had an inadequate response to treatment with a systemic corticosteroid (e.g., prednisone, methylprednisolone), azathioprine, 6-mercaptopurine, cyclosporine, or tacrolimus (Prograf) for at least 2 months. Dosing in ulcerative colitis in adults: 5 mg/kg as an IV infusion followed with additional similar doses at 2 and 6 weeks after the first infusion and then every 8 weeks thereafter. If the patient has an inadequate response to 5 mg/kg, the dose can be increased gradually up to a maximum of 10 mg/kg. In patients who lose response before the next dose is scheduled, the interval can be decreased to every 7, 6, 5, or 4 weeks or the dose per infusion can be increased. The maximum dose is 10 mg/kg and the shortest interval is every 4 weeks. Initial approval/extended approval: For induction therapy in active ulcerative colitis, initial approval is for 3 doses of infliximab given at weeks 0, 2 and 6. Patients are evaluated for response after the first 3 doses, about week 14, and for further maintenance therapy. If patients do not respond to the 3 infusions, further treatment with infliximab is not recommended. Approve for an additional 12 months of therapy if the patient has responded as determined by the prescribing gastroenterologist. The patient may not have a full response after 3 doses, but there should be some response. If the response is inadequate the dose can be gradually increased up to 10 mg/kg or a lesser dose can be given at a more frequent interval. Duration of therapy in ulcerative colitis: indefinite if the patient is responding. It is well accepted that maintenance therapy is indicated if there is a response to induction therapy. Labs/Diagnostics required: Patient should be screened for latent tuberculosis (TB) infection prior to starting therapy and then every year while on infliximab. Screening may include any of the following: history, tuberculin skin testing, interferon gamma release assay (IGRA), chest x-ray. In general, patients with latent TB infection should begin preventive therapy before starting infliximab. Waste management: There is no overfill in the infliximab vials. For patients who have already been started on infliximab, the dose should be calculated and the number of vials needed assessed. Usually the dose should be rounded to the next whole number of vials to avoid wasting unused medication left in the vial. Dose 5 mg/kg Body weight 61 kg or less Body weight 62 to 81 kg Body weight 82 to 100 kg Number of vials 3 4 5 Exclusions: same for all indications. . See below. 12/09/2009 12 Infliximab (Remicade) 7. Juvenile idiopathic arthritis (JIA) or juvenile rheumatoid arthritis (JRA), polyarticular course (regardless of type of onset). Indicated for the treatment of polyarticular course JIA or JRA in patients who meet all of the following criteria. [NOT FDA-APPROVED INDICATION] Infliximab is prescribed by a rheumatologist, and Patient has had an inadequate response to at least 2 months of therapy with methotrexate and Exceptions to having tried methotrexate can be made in the following circumstances: Infliximab will be given in combination with methotrexate or Patient has an absolute contraindication to MTX (e.g., pregnancy, breast feeding, alcoholic liver disease, immunodeficiency syndrome, blood dyscrasias). Patient has tried adalimumab (Humira) or etanercept (Enbrel) and had an inadequate response after at least 2 months of therapy or was intolerant to one of these other TNF antagonists. Note: adalimumab and etanercept are FDA approved in the treatment of JIA. Dosing in JIA/JRA: 3 to 6 mg/kg IV infusion followed by additional similar doses at 2 and 6 weeks after the first infusion, then every 8 weeks thereafter. The maximum maintenance dose is 10 mg/kg/infusion. Infliximab is FDA approved for the treatment of Crohn’s disease in children. The recommended dose in children with Crohn’s disease is 5 mg/kg given as an IV induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks. Other dosing regimens are not FDA approved in Crohn’s disease in children. Initial approval/extended approval: Initial approval is for 3 doses which is usually for doses at weeks 0, 2, and 6. Patients are evaluated for response after the third dose, at about week 12, and for further maintenance therapy. After 3 doses, approve for an additional 12 months of therapy if the patient has responded (e.g., has improvement in limitation of motion; less joint pain or tenderness; decreased duration of morning stiffness or fatique; improved function or activities of daily living; reduced dosage of corticosteroids), as determined by the prescribing rheumatologist. The patient may not have a full response by about week 12, but there should be some response. Duration of therapy in JIA/JRA: indefinite in patients who are responding. Labs/Diagnostics required: Patient should be screened for latent tuberculosis (TB) infection prior to starting therapy and then every year while on infliximab. Screening may include any of the following: history, tuberculin skin testing, interferon gamma release assay (IGRA), and/or chest x-ray. In general, patients with latent TB infection should begin preventive therapy before starting infliximab. Waste management: There is no overfill in the infliximab vials. The initial dose in JIA/JRA is 3 to 6 mg/kg. For patients who are already on infliximab, the dose should be 12/09/2009 13 Infliximab (Remicade) calculated and the number of vials needed assessed. Usually the dose should be rounded to the next whole number of vials to avoid wasting unused medication left in the vial. Dose 3 mg/kg Body weight less than 70 kg Body weight 70 to 100 kg Dose 5 mg/kg Body weight 61 kg or less Body weight 62 to 81 kg Body weight 82 to 100 kg Dose 6 mg/kg Body weight 50 kg or less Body weight 51 to 67 kg Body weight 68 to 84 kg Body weight 85 to 100 kg Number of vials 2 3 3 4 5 3 4 5 6 Exclusions: same for all indications. See below. 8. Undifferentiated spondyloarthropathy/spondyloarthritis in adults: Indicated for the treatment of undifferentiated spondyloarthropathy/spondyloarthritis in patients who meet all of the following criteria. [NOT FDA-APPROVED INDICATION] Infliximab is prescribed by a rheumatologist, and Patient has had an inadequate response to therapy with 2 different oral nonsteroidal antiinflammatory drugs (NSAID) that have been tried in about 1 month (for example, try one NSAID for 10 to 14 days and try a second for 10 to 14 days). Some examples of NSAIDs are naproxen, diclofenac, ibuprofen, ketoprofen, oxaprozin, piroxicam. Patient has tried a self-administered TNF antagonist, etanercept (Enbrel) or adalimumab (Humira) and had an inadequate response after at least 2 months of therapy or was intolerant to one of these other TNF antagonists. Dosing in undifferentiated spondyloarthropathy/spondyloarthritis: 5 mg/kg IV infusion followed by additional similar doses at 2 and 6 weeks after the first infusion, then every 8 weeks thereafter. Initial approval/extended approval: Initial approval is for 3 doses which is usually for doses at weeks 0, 2, and 6. Patients are evaluated for response after the third dose, at about week 12, and for further maintenance therapy. If patients do not respond to the 3 infusions, further treatment with infliximab is not recommended. Approve for an additional 12 months of therapy if the patient has responded as determined by the prescribing rheumatologist. The patient may not have a full response by about week 12, but there should be some response. Duration of therapy in undifferentiated spondyloarthropathy/spondyloarthritis: indefinite if the patient is responding. 12/09/2009 14 Infliximab (Remicade) Labs/Diagnostics required: Patient should be screened for latent tuberculosis (TB) infection prior to starting therapy and then every year while on infliximab. Screening may include any of the following: history, tuberculin skin testing, interferon gamma release assay (IGRA), chest x-ray. In general, patients with latent TB infection should begin preventive therapy before starting infliximab. Waste management: There is no overfill in the infliximab vials. For patients who are already on infliximab, the dose should be calculated and the number of vials needed assessed. Usually the dose should be rounded to the next whole number of vials to avoid wasting unused medication left in the vial. Dose 5 mg/kg Body weight 61 kg or less Body weight 62 to 81 kg Body weight 82 to 100 kg Number of vials 3 4 5 Exclusions: same for all indications. See below. 9. Still’s disease in adults (systemic-onset RA in adults, the disease may have begun in childhood): Indicated for the treatment of Still’s disease in patients who meet all of the following criteria. [NOT FDA-APPROVED INDICATION] Infliximab is prescribed by a rheumatologist, and Patient has tried a corticosteroid (e.g., prednisone, methylprednisolone) and has had an inadequate response to one non-biologic DMARD such as methotrexate (see DMARD table) given for at least 2 months or was intolerant to a non-biologic DMARD. Dosing in Still’s disease: 3 to 5 mg/kg IV infusion followed by additional similar doses at 2 and 6 weeks after the first infusion, then every 8 weeks thereafter. Initial approval/extended approval: Initial approval is for 3 doses which is usually for doses at weeks 0, 2, and 6. Patients are evaluated for response after the third dose, at about week 12, and for further maintenance therapy. If patients do not respond to the 3 infusions, further treatment with infliximab is not recommended. Approve for an additional 12 months of therapy if the patient has responded as determined by the prescribing rheumatologist. The patient may not have a full response by about week 12, but there should be some response. Duration of therapy in Still’s disease: indefinite if the patient is responding. Labs/Diagnostics required: Patient should be screened for latent tuberculosis (TB) infection prior to starting therapy and then every year while on infliximab. Screening may include any of the following: history, tuberculin skin testing, interferon gamma release assay 12/09/2009 15 Infliximab (Remicade) (IGRA), chest x-ray. In general, patients with latent TB infection should begin preventive therapy before starting infliximab. Waste management: There is no overfill in the infliximab vials. For patients who are already on infliximab, the dose should be calculated and the number of vials needed assessed. Usually the dose should be rounded to the next whole number of vials to avoid wasting unused medication left in the vial. Dose 3 mg/kg Body weight less than 70 kg Body weight 70 to 100 kg Dose 5 mg/kg Body weight 61 kg or less Body weight 62 to 81 kg Body weight 82 to 100 kg Number of vials 2 3 3 4 5 Exclusions: same for all indications. See below. 10. SAPHO (synovitis, acne, pustulosis, hyperostosis, osteotis) syndrome in adults: Indicated for the treatment of SAPHO in patients who meet all of the following criteria. [NOT FDA-APPROVED INDICATION] Infliximab is prescribed by a rheumatologist, and Patient has had an inadequate response to an NSAID and at least one of the following: methotrexate, a systemic corticosteroid, sulfasalazine, or cyclosporine. Some examples of NSAIDs are naproxen, diclofenac, ibuprofen, ketoprofen, oxaprozin, piroxicam (see NSAID table). Dosing in SAPHO: 3 to 5 mg/kg IV infusion followed by additional similar doses at 2 and 6 weeks after the first infusion, then every 6 weeks thereafter. Initial approval/extended approval: Initial approval is for 3 doses which is usually for doses at weeks 0, 2, and 6. Patients are evaluated for response after the third dose, at about week 12, and for further maintenance therapy. If patients do not respond to the 3 infusions, further treatment with infliximab is not recommended. Approve for an additional 12 months of therapy if the patient has responded (such as improvement of chest pain, bone pain, skin lesions or other symptoms) as determined by the prescribing rheumatologist. The patient may not have a full response by about week 12, but there should be some response. Duration of therapy in SAPHO: indefinite if the patient is responding. Labs/Diagnostics required: Patient should be screened for latent tuberculosis (TB) infection prior to starting therapy and then every year while on infliximab. Screening may include any of the following: history, tuberculin skin testing, interferon gamma release assay 12/09/2009 16 Infliximab (Remicade) (IGRA), chest x-ray. In general, patients with latent TB infection should begin preventive therapy before starting infliximab. Waste management: There is no overfill in the infliximab vials. For patients who are already on infliximab, the dose should be calculated and the number of vials needed assessed. Usually the dose should be rounded to the next whole number of vials to avoid wasting unused medication left in the vial. Dose 3 mg/kg Body weight less than 70 kg Body weight 70 to 100 kg Dose 5 mg/kg Body weight 61 kg or less Body weight 62 to 81 kg Body weight 82 to 100 kg Number of vials 2 3 3 4 5 Exclusions: same for all indications. See below. 11. Indeterminate colitis in adults: Indicated in refractory indeterminate colitis in adults who meet all of the following criteria. (Indeterminate colitis is defined as colitis that cannot be classified with certainty as either ulcerative colitis or Crohn’s disease). [NOT FDAAPPROVED INDICATION] Infliximab is prescribed by a gastroenterologist and Patient has tried a systemic corticosteroid and has had an inadequate response to mesalamine (e.g., Asacol, Pentasa, Lialda, Apriso) AND azathioprine or 6-mercaptopurine. Dosing in indeterminate colitis in adults: 5 mg/kg as an IV infusion followed with additional similar doses at 2 and 6 weeks after the first infusion and then every 8 weeks thereafter. Initial approval/extended approval: Initial approval is for 4 doses which is usually for doses at weeks 0, 2, 6 and 14. Patients are evaluated for response after the first 4 doses, about week 20, and for further maintenance therapy. If patients do not respond to the 4 infusions, further treatment with infliximab is not recommended. Approve for an additional 12 months of therapy if the patient has responded as determined by the prescribing physician. The patient may not have a full response by about week 20 but there should be some response. If the response is inadequate the dose can be gradually increased up to 10 mg/kg or a lesser dose can be given at a more frequent interval. The maximum dose is 10 mg/kg and the shortest interval is every 4 hours. Duration of therapy in indeterminate colitis: indefinite if the patient is responding. Labs/Diagnostics required: Patient should be screened for latent tuberculosis (TB) infection prior to starting therapy and then every year while on infliximab. Screening may 12/09/2009 17 Infliximab (Remicade) include any of the following: history, tuberculin skin testing, interferon gamma release assay (IGRA), chest x-ray. In general, patients with latent TB infection should begin preventive therapy before starting infliximab. Waste management: There is no overfill in the infliximab vials. For patients who have already been started on infliximab, the dose should be calculated and the number of vials needed assessed. Usually the dose should be rounded to the next whole number of vials to avoid wasting unused medication left in the vial. Dose 5 mg/kg Body weight 61 kg or less Body weight 62 to 81 kg Body weight 82 to 100 kg Number of vials 3 4 5 Exclusions: same for all indications. . See below. 12. Pouchitis in adults: Indicated in patients with active disease and to maintain remission in patients who meet all of the following criteria. [NOT FDA-APPROVED INDICATION] Infliximab is prescribed by a gastroenterologist and Patient has tried therapy with an antibiotic (metronidazole [Flagyl], ciprofloxacin), probiotic (live microorganisms), corticosteroid enema (e.g., hydrocortisone [Cortifoam, Cortenema]), or mesalamine (Rowasa) enema. Dosing in pouchitis in adults: 5 mg/kg as an induction regimen at 0, 2, and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter. Initial approval/extended approval: for inducing remission in active pouchitis, initial approval is for 3 doses of infliximab given at weeks 0, 2 and 6. Patients are evaluated for response after the first 3 doses, about week 14, and for further maintenance therapy. If patients do not respond to the 3 infusions, further treatment with infliximab is not recommended. Approve for an additional 12 months of therapy if the patient has responded as determined by the prescribing gastroenterologist. Duration of therapy in pouchitis: indefinite if the patient is responding. It is well accepted that maintenance therapy is indicated if there is a response to induction therapy. Labs/Diagnostics required: Patient should be screened for latent tuberculosis (TB) infection prior to starting therapy and then every year while on infliximab. Screening may include any of the following: history, tuberculin skin testing, interferon gamma release assay (IGRA), chest x-ray. In general, patients with latent TB infection should begin preventive therapy before starting infliximab. 12/09/2009 18 Infliximab (Remicade) Waste management: There is no overfill in the infliximab vials. For patients who have already been started on infliximab, the dose should be calculated and the number of vials needed assessed. Usually the dose should be rounded to the next whole number of vials to avoid wasting unused medication left in the vial. Dose 5 mg/kg Body weight 61 kg or less Body weight 62 to 81 kg Body weight 82 to 100 kg Number of vials 3 4 5 Exclusions: same for all indications. . See below. 13. Pyoderma gangrenosum in adults: Indicated in refractory pyoderma gangrenosum in adults who meet all of the following criteria. [NOT FDA-APPROVED INDICATION] Infliximab is prescribed by a gastroenterologist, dermatologist, rheumatologist, or oncologist and Patient has had an inadequate response to treatment with a systemic corticosteroid (e.g., prednisone, methylprednisolone) or cyclosporine for at least 2 months. Dosing in pyoderma gangrenosum in adults: 5 mg/kg as an IV infusion followed with additional similar doses at 2 and 6 weeks after the first infusion and then every 8 weeks thereafter. Initial approval/extended approval: Initial approval is for 4 doses which is usually for doses at weeks 0, 2, 6 and 14. Patients are evaluated for response after the first 4 doses, about week 20, and for further maintenance therapy. If patients do not respond to the 4 infusions, further treatment with infliximab is not recommended. Approve for an additional 12 months of therapy if the patient has responded as determined by the prescribing gastroenterologist, dermatologist, rheumatologist, or oncologist. The patient may not have a full response by about week 20 but there should be some response. If the response is inadequate the dose can be gradually increased up to 10 mg/kg or a lesser dose can be given at a more frequent interval. The maximum dose is 10 mg/kg and the shortest interval is every 4 hours. Duration of therapy in pyoderma gangrenosum: indefinite if the patient is responding. Labs/Diagnostics required: Patient should be screened for latent tuberculosis (TB) infection prior to starting therapy and then every year while on infliximab. Screening may include any of the following: history, tuberculin skin testing, interferon gamma release assay (IGRA), chest x-ray. In general, patients with latent TB infection should begin preventive therapy before starting infliximab. 12/09/2009 19 Infliximab (Remicade) Waste management: There is no overfill in the infliximab vials. For patients who have already been started on infliximab, the dose should be calculated and the number of vials needed assessed. Usually the dose should be rounded to the next whole number of vials to avoid wasting unused medication left in the vial. Dose 5 mg/kg Body weight 61 kg or less Body weight 62 to 81 kg Body weight 82 to 100 kg Number of vials 3 4 5 Exclusions: same for all indications. . See below. 14. Enterovesical fistulas (bowel to bowel, bowel to bladder, bowel to urethra) in adults with Crohn’s disease. Indicated in refractory enterovesical fistulas in adults with Crohn’s disease who meet all of the following criteria. [NOT FDA-APPROVED INDICATION] Infliximab is prescribed by a gastroenterologist and Patient has had an inadequate response to treatment with azathioprine, 6-mercaptopurine, mycophenolate mofetil (Cellcept), cyclosporine, or tacrolimus (Prograf). Dosing in enterovesical fistulas in adults: 5 mg/kg as an IV infusion followed with additional similar doses at 2 and 6 weeks after the first infusion and then every 8 weeks thereafter. Initial approval/extended approval: Initial approval is for 4 doses which is usually for doses at weeks 0, 2, 6 and 14. Patients are evaluated for response after the first 4 doses, about week 20, and for further maintenance therapy. If patients do not respond to the 4 infusions, further treatment with infliximab is not recommended. Approve for an additional 12 months of therapy if the patient has responded as determined by the prescribing physician. The patient may not have a full response by about week 20 but there should be some response. If the response is inadequate the dose can be gradually increased up to 10 mg/kg or a lesser dose can be given at a more frequent interval. The maximum dose is 10 mg/kg and the shortest interval is every 4 hours. Duration of therapy in enterovesical fistulas: indefinite if the patient is responding. Labs/Diagnostics required: Patient should be screened for latent tuberculosis (TB) infection prior to starting therapy and then every year while on infliximab. Screening may include any of the following: history, tuberculin skin testing, interferon gamma release assay (IGRA), chest x-ray. In general, patients with latent TB infection should begin preventive therapy before starting infliximab. Waste management: There is no overfill in the infliximab vials. For patients who have already been started on infliximab, the dose should be calculated and the number of vials 12/09/2009 20 Infliximab (Remicade) needed assessed. Usually the dose should be rounded to the next whole number of vials to avoid wasting unused medication left in the vial. Dose 5 mg/kg Body weight 61 kg or less Body weight 62 to 81 kg Body weight 82 to 100 kg Number of vials 3 4 5 Exclusions: same for all indications. See below. 15. Hidradenitis suppurativa: Indicated for the treatment of moderate to severe hidradenitis suppurativa in patients who meet all of the following criteria. [NOT FDAAPPROVED INDICATION] Infliximab is prescribed by a dermatologist, and The patient is 16 years of age and older, and Patient has had an inadequate response to at least one course of topical or systemic antibiotic therapy, intralesional or oral corticosteroids, or isotretinoin and Patient has tried a self-administered TNF antagonist, etanercept (Enbrel) or adalimumab (Humira) for at least 2 months and had an inadequate response or was intolerant to one of these other TNF antagonists. Dosing in hidradenitis suppurativa: 5 mg/kg IV infusion followed by additional similar doses at 2 and 6 weeks after the first infusion, then every 8 weeks thereafter. Initial approval/extended approval: Initial approval is for 3 doses which is usually for doses at weeks 0, 2, and 6. Patients are evaluated for response after the third dose, at about week 12, and for further maintenance therapy. If patients do not respond to the 3 infusions, further treatment with infliximab is not recommended. Approve for an additional 12 months of therapy if the patient has responded as determined by the prescribing physician. The patient may not have a full response by about week 12, but there should be some response. Duration of therapy in RA: indefinite if the patient is responding. Labs/Diagnostics required: Patient should be screened for latent tuberculosis (TB) infection prior to starting therapy and then every year while on infliximab. Screening may include any of the following: history, tuberculin skin testing, interferon gamma release assay (IGRA), chest x-ray. In general, patients with latent TB infection should begin preventive therapy before starting infliximab. Waste management: There is no overfill in the infliximab vials. For patients who are already on infliximab, the dose should be calculated and the number of vials needed assessed. 12/09/2009 21 Infliximab (Remicade) Usually the dose should be rounded to the next whole number of vials to avoid wasting unused medication left in the vial. Dose 5 mg/kg Body weight 61 kg or less Body weight 62 to 81 kg Body weight 82 to 100 kg 3 4 5 Exclusions: same for all indications. See below. Exclusions: Concomitant use with anakinra (Kineret), any other TNF antagonist (such as etanercept, adalimumab, golimumab [Simponi], certolizumab pegol [Cimzia]), abatacept (Orencia), rituximab (Rituxan), natalizumab (Tysabri), alefacept (Amevive), or ustekinumab (Stelara). Doses > 5 mg/kg should not be administered in patients with congestive heart failure. Contraindicated in patients with moderate or severe heart failure (New York Heart Association class III-IV) with reduced ejection fraction. Intra-articular administration is not FDA approved. Infliximab is contraindicated in patients with significant active infections. Wegener’s granulomatosis. REFERENCES Remicade for intravenous infusion [package insert]. Malvern, PA: Centocor, Inc; November 2009. Rheumatoid Arthritis St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: A randomized, controlled trial. Arthritis Rheum. 2004;50:3432-3443. Lipsky PE, van der Heijde DM, St Clair EW, et al; for the Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med. 2000;343:1594-1602. Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932-1939. Maini RN, Breedveld FC, Kalden JR, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50:1051-1065. Smolen JS, Han C, van der Heijde DM, et al. Radiographic changes in rheumatoid arthritis patients attaining different disease activity states with methotrexate monotherapy and infliximab plus methotrexate: the impacts of remission and TNFblockade. Ann Rheum Dis. 2009;68:823-827. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381-3390. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2007;146:406-415. van der Bijl AE, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. Infliximab and methotrexate as induction therapy in patients with early rheumatoid arthritis. Arthritis Rheum. 2007;56:2129-2134. Schiff M, Keiserman M, Codding C, et al. Efficacy and safety of abatacept or infliximab versus placebo in ATTEST: a phase III, multicenter, randomized, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67:1096-1103. Furst DE, Keystone EC, Kirkham B, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2008. Ann Rheum Dis. 2008;67 Suppl 3:iii2-25. Saag KG, Teng GG, Patkar NM, et al; American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. 12/09/2009 22 Infliximab (Remicade) Arthritis Rheum. 2008;59:762-784. Available at: http://www.rheumatology.org/publications/guidelines/recommendations.asp?aud=mem Accessed 08/05/2009. American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis. Arthritis Rheum. 2002;46:328-346. Available at http://www.rheumatology.org/publications/guidelines/raguidelines02.asp?aud=mem. Accessed on 08/05/2009. van Vollenhoven RF. How to dose infliximab in rheumatoid arthritis: new data on a serious issue. Ann Rheum Dis. 2009;68:1237-1239. van Vollenhoven RF, Ernestam S, Beborek P, et al. Addition of infliximab compared with addition of sulfasalazine and hydrozychloroquive to methotrexate in patients with eary rheumatoid arthritis (Swefort) trial): 1-year results of a randomized trial. Lancet. 2009:374:459-466. Lutt JR, Deodhar A. Rheumatoid arthritis: strategies in the management of patients showing an inadequate response to TNFalpha antagonists. Drugs. 2008;68:591-606. Flendrie M, Creemers MC, van Riel PL. Titration of infliximab treatment in rheumatoid arthritis patients based on response patterns. Rheumatology (Oxford). 2007;46:146-149. Rahman MU, Strusberg I, Geusens P, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:1233-1238. St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451-1459. Sidiropoulos P, Bertsias G, Kritikos HD, et al. Infliximab treatment for rheumatoid arthritis, with dose titration based on the Disease Activity Score: dose adjustments are common but not always sufficient to assure sustained benefit. Ann Rheum Dis. 2004;63:144-148. Durez P, Van den Bosch F, Corluy L, et al. A dose adjustment in patients with rheumatoid arthritis not optimally responding to a standard dose of infliximab of 3 mg/kg every 8 weeks can be effective: a Belgian prospective study. Rheumatology (Oxford). 2005;44:465-468. Strangfeld A, Hierse F, Kekow J, et al. Comparative effectiveness of TNFalpha inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis. 2009 Jan 6. [Epub ahead of print] Flendrie M, Creemers MC, Welsing PM, van Riel PL. The influence of previous and concomitant leflunomide on the efficacy and safety of infliximab therapy in patients with rheumatoid arthritis; a longitudinal observational study. Rheumatology (Oxford). 2005;44:472-478. Hansen KE, Cush J, Singhal A, et al. The safety and efficacy of leflunomide in combination with infliximab in rheumatoid arthritis. Arthritis Rheum. 2004;51:228-232. Hyrich KL, Symmons DP, Watson KD, Silman AJ; British Society for Rheumatology Biologics Register. Comparison of the response to infliximab or etanercept monotherapy with the response to cotherapy with methotrexate or another diseasemodifying antirheumatic drug in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54:1786-1794. Psoriatic Arthritis Gottlieb A, Korman NJ, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol. 2008;58:851-864. Ritchlin CT, Kavanaugh A, Gladman DD, et al. Treatment recommendations for psoriatic arthritis. Ann Rheum Dis. 2009;68:1387-1394. Antoni C, Krueger GG, de Vlam K, et al; IMPACT 2 Trial Investigators. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis. 2005;64:1150-1157. Kavanaugh A, Krueger GG, Beutler A, et al; for the IMPACT Study Group. Infliximab maintains a high degree of clinical response in patients with active psoriatic arthritis through one year of treatment: results from the IMPACT 2 trial. Ann Rheum Dis. 2007;66:498-505. van der Heijde D, Kavanaugh A, Gladman DD, et al. Infliximab inhibits progression of radiographic damage in patients with active psoriatic arthritis through one year of treatment: Results from the induction and maintenance psoriatic arthritis clinical trial 2. Arthritis Rheum. 2007;56:2698-2707. Kavanaugh A, Antoni C, Krueger GG, et al. Infliximab improves health-related quality of life and physical function in patients with psoriatic arthritis. Ann Rheum Dis. 2006;65:471-477. Antoni CE, Kavanaugh A, Kirkham B, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum. 2005;52:1227-1236. Kavanaugh A, Antoni CE, Gladman DD, et al. The Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT): Results of radiographic analyses after 1 year. Ann Rheum Dis. 2006;65:1038-1043. Antoni CE, Kavanaugh A, van der Heijde D, et al. Two-year efficacy and safety of infliximab treatment in patients with active psoriatic arthritis: findings of the Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT). J Rheumatol. 2008;35:869-876. Ankylosing Spondylitis 12/09/2009 23 Infliximab (Remicade) Zochling J, van der Heijde D, Burgos-Vargas R, et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2006;65:442-452. Zochling J, van der Heijde D, Dougados M, et al. Current evidence for the management of ankylosing spondylitis a systematic literature review for the ASAS/EULAR management recommendations in ankylosing spondylitis. Ann Rheum Dis. 2006;65:423-432. Zochling J, Braun J. Quality indicators, guidelines and outcome measures in ankylosing spondylitis. Clin Exp Rheumatol. 2007;25(6 Suppl 47):147-152. van der Heijde D, Landewé R, Baraliakos X, et al; Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy Study Group. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum. 2008;58:3063-3070. van der Heijde D, Dijkmans B, Geusens P, et al; Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy Study Group. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum. 2005;52:582-591. Braun J, Landewe R, Hermann KG, et al; ASSERT Study Group. Major reduction in spinal inflammation in patients with ankylosing spondylitis after treatment with infliximab: results of a multicenter, randomized, double-blind, placebocontrolled magnetic resonance imaging study. Arthritis Rheum. 2006;54:1646-1652. Braun J, Deodhar A, Dijkmans B, et al; Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy Study Group. Efficacy and safety of infliximab in patients with ankylosing spondylitis over a two-year period. Arthritis Rheum. 2008;59:1270-1278. Braun J, Brandt J, Listing J, et al. Treatment of active ankylosing spondylitis with infliximab: a randomized controlled multicentre trial. Lancet. 2002;359:1187-1193. Braun J, Baraliakos X, Listing J, et al. Persistent clinical efficacy and safety of anti-tumour necrosis factor alpha therapy with infliximab in patients with ankylosing spondylitis over 5 years: evidence for different types of response. Ann Rheum Dis. 2008;67:340-345. Breban M, Ravaud P, Claudepierre P, et al; French Ankylosing Spondylitis Infliximab Network. Maintenance of infliximab treatment in ankylosing spondylitis: results of a one-year randomized controlled trial comparing systematic versus ondemand treatment. Arthritis Rheum. 2008;58:88-97. Plaque Psoriasis Kalb RE, Bagel J, Korman NJ, et al.; National Psoriasis Foundation. Treatment of intertriginous psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2009;60:120-124. Callen JP, Krueger GG, Lebwohl M, et al. AAD consensus statement on psoriasis therapies. J Am Acad Dermatol. 2003;49:897-899. Pariser DM, Bagel J, Gelfand JM, et al; National Psoriasis Foundation. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242. Menter A, Griffiths CEM. Current and future management of psoriasis. Lancet. 2007;370:272-284. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. Feldman SR, Koo JY, Menter A, et al. Decision points for the initiation of systemic treatment for psoriasis. J Am Acad Dermatol. 2005;53:101-107. Reich K, Nestle FO, Papp K, et al; EXPRESS study investigators. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367-1374. Reich K, Nestle FO, Papp K, et al. Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate-to-severe psoriasis: a randomized controlled trial. Br J Dermatol. 2006;154:1161-1168. Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56:31.e1-15. Gottlieb AB, Evans R, Li S, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51:534-542. Rich P, Griffiths CE, Reich K, et al. Baseline nail disease in patients with moderate to severe psoriasis and response to treatment with infliximab during 1 year. J Am Acad Dermatol. 2008;58:224-231. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2009 Oct 6. [Epub ahead of print] Inflammatory Bowel Disease 12/09/2009 24 Infliximab (Remicade) Lichtenstein GR, Hanauer SB, Sandborn WJ; and The Practice Parameters Committee of the American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol. 2009;104:465-483. Clark M, Colombel JF, Feagan BC, et al. American gastroenterological association consensus development conference on the use of biologics in the treatment of inflammatory bowel disease, June 21-23, 2006. Gastroenterology. 2007;133:312339. Lichtenstein GR, Abreu MT, Cohen R, et al; American Gastroenterological Association. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:940-987. Lichtenstein GR, Abreu MT, Cohen R, et al; American Gastroenterological Association. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:935-939. Sandborn WJ, Fazio VW, Feagan BG, et al; American Gastroenterological Association Clinical Practice Committee. AGA technical review on perianal Crohn's disease. Gastroenterology. 2003;125:1508-1530. Baumgart DC Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641-1657. Peyrin-Biroulet L, Deltenre P, de Suray N, et al. Efficacy and safety of tumor necrosis factor antagonists in Crohn's disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2008;6:644-653. Parsi MA, Lashner BA, Achkar JP, et al. Type of fistula determines response to infliximab in patient with fistulous Crohn’s disease. Am J Gastroenterol. 2004;99:445-449. Barrie A, Regueiro M. Biologic therapy in the management of extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:1424-1429. Ferrante M, D'Haens G, Dewit O, et al; on behalf of the Belgian IBD Research Group. Efficacy of infliximab in refractory pouchitis and Crohn's disease-related complications of the pouch: A Belgian case series. Inflamm Bowel Dis. 2009 Jul 27. [Epub ahead of print] Colombel JF, Ricart E, Loftus EV Jr, et al. Management of Crohn's disease of the ileoanal pouch with infliximab. Am J Gastroenterol. 2003;98:2239-2244. Viscido A, Habib FI, Kohn A, et al. Infliximab in refractory pouchitis complicated by fistulae following ileo-anal pouch for ulcerative colitis. Aliment Pharmacol Ther. 2003;17:1263-1271. Pardi DS, D’Haens G, Shen B, et al. Clinical guidelines for the management of pouchitis. Inflamm Bowel Dis. 2009;15;1424-1431/ Sandborn WJ, Rutgeerts P, Feagan BG, et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology. 2009;137:1250-1260. Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn's disease: a review. Am J Gastroenterol. 2009;104:760-767. Regueiro M, Siemanowski B, Kip KE, Plevy S. Infliximab dose intensification in Crohn's disease. Inflamm Bowel Dis. 2007;13:1093-1099. Magro F, Bastos R, Marques M, Costa Santos C. Infliximab dose intensification by shortening infusion intervals. Inflamm Bowel Dis. 2008;14:432-434. Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther. 2009;30:210-226. Hommes DQ. Risks and benefits of biologic therapy for IBD. J Pediatr Gastroenterol Nutr. 2009;48 Suppl 2:S52-S53. . D'Haens G, Baert F, van Assche G, et al; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. 2008;371:660-667. Lemann M, Mary JY, Duclos B, et al; Groupe d'Etude Therapeutique des Affections Inflammatoires du Tube Digestif (GETAID). Infliximab plus azathioprine for steroid-dependent Crohn's disease patients: a randomized placebo-controlled trial. Gastroenterology. 2006;130:1054-1061. Stephens MC, Shepanski MA, Mamula P, et al. Safety and steroid-sparing experience using infliximab for Crohn’s disease at a pediatric inflammatory bowel disease center. Am J Gastroenterol. 2003;98:104-111. Van Assche G, Magdelaine-Beuzelin C, D'Haens G, et al. Withdrawal of immunosuppression in Crohn's disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology. 2008;134:1861-1868. Akobeng AK. Review article: the evidence base for interventions used to maintain remission in Crohn's disease. Aliment Pharmacol Ther. 2008;27:11-18. McGinnis JK, Murray KF. Infliximab for ulcerative colitis in children and adolescents. J Clin Gastroenterol. 2008;42:875879. Rutgeerts P, Sandborn WJ, Feagan BG, eta l. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. Jarnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005;128:1805-1811. 12/09/2009 25 Infliximab (Remicade) Feagan BG, Reinisch W, Rutgeerts P, ET AL. The effects of infliximab therapy on health-related quality of life in ulcerative colitis patients. Am J Gastroenterol. 2007;102:794-802. Fanjiang G, Russell GH, Katz AJ. Short- and long-term response to and weaning from infliximab therapy in pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr. 2007;44:312-317. JIA OR JRA [NOT FDA-APPROVED INDICATION] Lahdenne P, Vahasalo P, Honkanen V. Infliximab or etanercept in the treatment of children with refractory juvenile idiopathic arthritis: an open label study. Ann Rheum Dis. 2003;62:245-247. Gerloni V, Pontikaki I, Gattinara M, et al. Efficacy of repeated intravenous infusions of an anti-tumor necrosis factor alpha monoclonal antibody, infliximab, in persistently active, refractory juvenile idiopathic arthritis: results of an open-label prospective study. Arthritis Rheum. 2005;52:548-553. Ruperto N, Lovell DJ, Cuttica R, et al; Paediatric Rheumatology International Trials Organisation; Pediatric Rheumatology Collaborative Study Group. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007;56:3096-3106. Tse SM, Burgos-Vargas R, Laxer RM. Anti-tumor necrosis factor alpha blockade in the treatment of juvenile spondylarthropathy. Arthritis Rheum. 2005;52:2103-2108. Ruperto N, Lovell DJ, Cuttica R, et al. Long-term efficacy and safety of infliximab plus methotrexate for the treatment of polyarticular course juvenile rheumatoid arthritis: Findings from an open-label treatment extension. Ann Rheum Dis. 2009 Apr 29. [Epub ahead of print] Undifferentiated spondyloarthropathy/spondyloarthritis [NOT FDA-APPROVED INDICATION] Davis JC, Mease PJ. Insights into the pathology and treatment of spondyloarthritis: from the bench to the clinic. Semin Arthritis Rheum. 2008;38:83-100. Haibel H, Rudwaleit M, Listing J, et al. Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis: Results of a twelve-week randomized, double-blind, placebo-controlled trial followed by an open-label extension up to week fifty-two. Arthritis Rheum. 2008;58:1981-1991. Brandt J, Khariouzov A, Listing J, et al. Successful short term treatment of patients with severe undifferentiated spondyloarthritis with the anti-tumor necrosis factor-alpha fusion receptor protein etanercept. J Rheumatol. 2004;31:531-538. Flagg SD, Meador R, Hsia E, et al. Decreased pain and synovial inflammation after etanercept therapy in patients with reactive and undifferentiated arthritis: an open-label trial. Arthritis Rheum. 2005;53:613-617. Van Den Bosch F, Kruithof E, Baeten D, et al. Randomized double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondyloarthropathy. Arthritis Rheum. 2002;46:755-765. Brandt J, Haibel H, Reddig J, et al. Successful short term treatment of severe undifferentiated spondyloarthropathy with the anti-tumor necrosis factor-alpha monoclonal antibody infliximab. J Rheumatol. 2002;29:118-122. Still’s disease in adults [NOT FDA-APPROVED INDICATION] Dilhuydy MS, Vatan R, Etienne G, et al. Prolonged efficacy of infliximab for refractory adult-onset Still’s disease. Clin Exp Rheumatol. 2005;23:121-122. Fautrel B, Sibilia J, Mariette X, et al. Tumour necrosis factor alpha blocking agents in refractory adult Still’s disease: an observational study of 20 cases. Ann Rheum Dis. 2005;64:262-266. Kokkinos A, Iliopoulos A, Greka P, et al. Successful treatment of refractory adult-onset Still’s disease with infliximab. A prospective, non-comparative series of four patients. Clin Rheumatol. 2004;23:45-49. Caramaschi P, Carletto A, Biasi D, Bambara LM. A case of adult onset Still’s disease treated with infliximab. Clin Exp Rheumatol. 2002;20:113. Kraetsch HG, Antoni C, Kalden JR, Manger B. Successful treatment of a small cohort of patients with adult onset Still’s disease with infliximab: first experiences. Ann Rheum Dis. 2001;60 Suppl 3:iii55-iii57. Cavagna L, Caporali R, Epis O, et al. Infliximab in the treatment of adult Still’s disease refractory to conventional therapy. Clin Exp Rheumatol. 2001;19:329-332. Kontzias A, Efthimiou P. Adult-onset Still's disease: pathogenesis, clinical manifestations and therapeutic advances. Drugs. 2008;68:319-337. SAPHO [NOT FDA-APPROVED INDICATION] Moll C, Hernández MV, Cañete JD, et al. Ilium osteitis as the main manifestation of the SAPHO syndrome: response to infliximab therapy and review of the literature. Semin Arthritis Rheum. 2008;37:299-306. Indeterminate colitis [NOT FDA-APPROVED INDICATION] Papadakis KA, Treyzon L, Abreu MT, et al. Infliximab in the treatment of medically refractory indeterminate colitis. Aliment Pharmacol Ther. 2003;18:741-747. Gornet JM, Couve S, Hassani Z, et al. Infliximab for refractory ulcerative colitis or indeterminate colitis: an open-label multicentre study. Aliment Pharmacol Ther. 2003;18:175-181. Bordeianou L, Kunitake H, Shellito P, Hodin R. Preoperative infliximab treatment in patients with ulcerative and indeterminate colitis does not increase rate of conversion to emergent and multistep abdominal surgery. Int J Colorectal Dis. 2009 Oct 2. [Epub ahead of print] Pyoderma gangrenosum [NOT FDA-APPROVED INDICATION] Reichrath J, Bens G, Bonowitz A, et al. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273-283. 12/09/2009 26 Infliximab (Remicade) Regueiro M, Valentine J, Plevy S, et al. Infliximab for treatment of pyoderma gangrenosum associated with inflammatory bowel disease. Am J Gastroenterol. 2003;98:1821-1826. Brooklyn TN, Dunnill GS, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, doubleblind placebo-controlled trial. Gut. 2006;55:505-509. Juillerat P, Christen-Zäch S, Troillet FX, et al. Infliximab for the treatment of disseminated pyoderma gangrenosum associated with ulcerative colitis. Case report and literature review. Dermatology. 2007;215:245-251. Enterovesical fistulas [NOT FDA-APPROVED INDICATION] Parsi MA, Lashner BA, Achkar JP, et al. Type of fistula determines response to infliximab in patients with fistulous Crohn's disease. Am J Gastroenterol. 2004;99:445-449. Hidradenitis suppurativa [NOT FDA-APPROVED INDICATION] Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561. ClinicalTrials.gov. Study to assess the safety and efficacy of infliximab to treat hidradenitis suppurtativa. Bethesda, MD: U.S. National Institutes of Health; November 21, 2008 (last update). Available at: http://clinicaltrials.gov/ct2/show/NCT00795574?term=hs2006&rank=1. Accessed on: November 17, 2009. Adams DR, Gordon KB, Devenyi AG, et al. Severe hidradenitis suppurativa treated with infliximab infusion. Arch Dermatol. 2003;139:1540-1542. Mekkes JR, Bos JD. Long-term efficacy of a single course of infliximab in hidradenitis suppurativa. Br J Dermatol. 2008;158:370-374. Moul DK, Korman NJ. The cutting edge. Severe hidradenitis suppurativa treated with adalimumab. Arch Dermatol. 2006;142:1110-1112. Yamauchi PS, Mau N. Hidradenitis suppurativa managed with adalimumab. J Drugs Dermatol. 2009;8:181-183. Blanco R, Martinex-Taboada VM, Villa I, et al. Long-term successful adalimumab therapy in severe hidradenitis suppurativa. Arch Dermatol. 2009;145:580-584. Cusack C, Buckley C. Etanercept: effective in the management of hidradenitis suppurativa. Br J Dermatol. 2006;154:726729. Giamarellos-Bourboulis EJ, Pelekanou E, et al. An open-label phase II study of the safety and efficacy of etanercept for the therapy of hidradenitis suppurativa. Br J Dermatol. 2008;158:567-572. Lee RA, Dommasch E, Treat J, et al. A prospective clinical trial of open-label etanercept for the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2009;60:565-573. Ileocolonic resection Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn's disease recurrence after ileal resection. Gastroenterology. 2009;136:441-450. Sorrentino D, Terrosu G, Avellini C, Maiero S. Infliximab with low-dose methotrexate for prevention of postsurgical recurrence of ileocolonic Crohn disease. Arch Intern Med. 2007 10;167:1804-1807. Yamamoto T, Umegae S, Matsumoto K. Impact of infliximab therapy after early endoscopic recurrence following ileocolonic resection of Crohn's disease: A prospective pilot study. Inflamm Bowel Dis. 2009;15:1460-1466. Familial Mediterranean Fever [NOT FDA-APPROVED INDICATION] Nakamura A, Matsuda M, Tazawa K, et al. Successful treatment with infliximab and low-dose methotrexate in a Japanese patient with familial Mediterranean fever. Intern Med. 2007;46:1247-1249. Ozgocmen S, Ozçakar L, Ardicoglu O, et al. Familial Mediterranean fever responds well to infliximab: single case experience. Clin Rheumatol. 2006;25:83-87. Yüksel S, Yalçinkaya F, Acar B, et al. Clinical improvement with infliximab in a child with amyloidosis secondary to familial Mediterranean fever. Rheumatology (Oxford). 2006;45:1307-1308. Daysal S, Akcil G, Goker B, et al. Infliximab therapy in a patient with familial Mediterranean fever and chronic hip arthritis. Arthritis Rheum. 2005;53:146-147. Brik R, Gepstein V, Shahar E, et al. Tumor necrosis factor blockade in the management of children with orphan diseases. Clin Rheumatol. 2007;26:1783-1785. Amyloidosis with renal involvement in adults [NOT FDA-APPROVED INDICATION] Keersmaekers T, Claes K, Kuypers DR, et al. Long-term efficacy of infliximab treatment for AA-amyloidosis secondary to chronic inflammatory arthritis. Ann Rheum Dis. 2009;68:759-761. Gottenberg JE, Merle-Vincent F, Bentaberry F, et al. Anti-tumor necrosis factor alpha therapy in fifteen patients with AA amyloidosis secondary to inflammatory arthritides: a followup report of tolerability and efficacy. Arthritis Rheum. 2003;48:2019-2024. Fernandez-Nebro A, Tomero E, Ortiz-Santamaria V, et al. Treatment of rheumatic inflammatory disease in 25 patients with secondary amyloidosis using tumor necrosis factor alpha antagonists. Am J Med. 2005;118:552-556. Abbreviations: BSA = body surface area anti-CCP antibodies = anti-citrullinated peptide antibodies 12/09/2009 27 Infliximab (Remicade) CRP = C-reactive protein DMARD = disease modifying antirheumatic drug FDA = Food and Drug Administration IGRA = interferon gamma release assay IV = intravenous mL = milliliter NSAID = Nonsteroidal anti-inflammatory drug TB = tuberculosis TNF = tumor necrosis factor Disease Modifying Antirheumatic Drugs (DMARDs). Generic Name Trade Name Traditional (Synthetic) DMARDs azathioprine (oral) generics, Imuran cyclosporine (oral) generics, Neoral, Sandimmune d-penicillamine (oral) Cuprimine, Depen gold compounds gold sodium thiomalate (injection) Myochrysine® auranofin (oral) Ridaura hydroxychloroquine (oral) generics, Plaquenil leflunomide (oral) generics, Arava methotrexate [MTX] (oral, injection) generics, Rheumatrex minocycline (oral) generics, Minocin® sulfasalazine (oral) generics, Azulfidine En-tabs, Azulfidine Biologic DMARDs abatacept (injection) Orencia adalimumab (injection) Humira anakinra (injection) Kineret certolizumab pegol (injection) Cimzia® etanercept (injection) Enbrel golimumab (injection) Simponi® infliximab (injection) Remicade rituximab (injection) Rituxan® tocilizumab (injection) Actemra® 12/09/2009 28 Infliximab (Remicade) Nonsteroidal anti-inflammatory drugs (NSAIDs) list. Generic name Brand name examples diclofenac epolamine topical patch Flector® Patch diclofenac potassium Cataflam® , generics; Zipsor™ diclofenac sodium Voltaren®, Voltaren XR®, generics diclofenac sodium topical gel Voltaren® Gel diclofenac sodium and misoprostol tablets Arthrotec etodolac Lodine®, Lodine XL®, generics fenoprofen Nalfon®, generics flurbiprofen Ansaid®, generics) ibuprofen* Motrin®, Advil®, generics ibuprofen tablets and caffeine supplement capsules IC 400™ Kit, IC 800™ Kit indomethacin Indocin®, Indocin SR®, generics ketoprofen Orudis®, generics ketoprofen sustained-release Oruvail®, generics ketorolac, tablets, injectable Toradol®, generics lansoprazole delayed-release capsules and Prevacid® NapraPAC™ 500 naproxen tablets meclofenamate generics mefenamic acid capsules Ponstel, generics meloxicam tablets Mobic, generics nabumetone Relafen®, generics naproxen Naprosyn®, generics naproxen delayed-release Naprosyn EC®, generics naproxen controlled-release Naprelan®, generics naproxen sodium* Anaprox®, Anaprox DS®, generics oxaprozin Daypro®, generics piroxicam Feldene®, generics sulindac Clinoril®, generics tolmetin generics *Available without a prescription. 12/09/2009 29