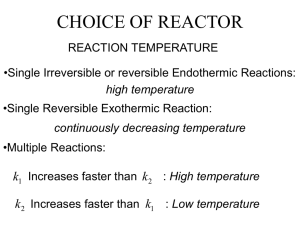
Form 20 - Heriot-Watt University
advertisement
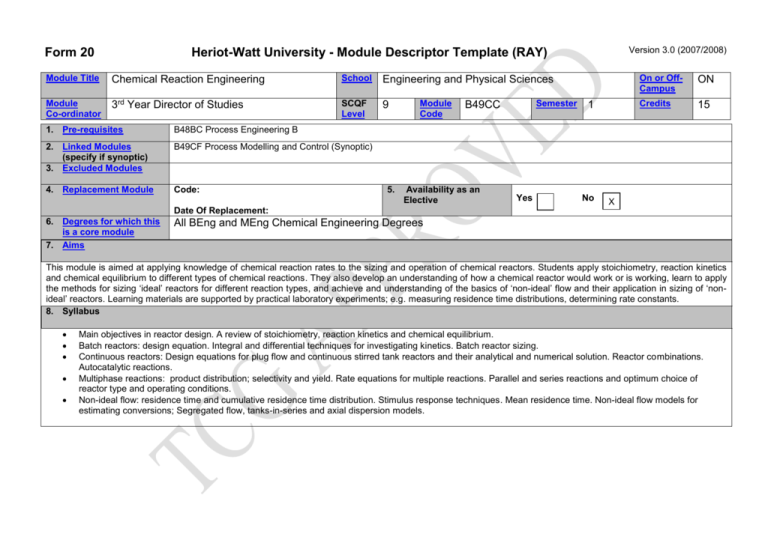
Form 20 Version 3.0 (2007/2008) Heriot-Watt University - Module Descriptor Template (RAY) Module Title Chemical Reaction Engineering School Engineering and Physical Sciences Module Co-ordinator 3rd Year Director of Studies SCQF Level 9 1. Pre-requisites B48BC Process Engineering B 2. Linked Modules (specify if synoptic) 3. Excluded Modules B49CF Process Modelling and Control (Synoptic) 4. Replacement Module Code: 5. Module Code Semester B49CC Availability as an Elective Yes 1 No On or OffCampus ON Credits 15 X Date Of Replacement: 6. Degrees for which this is a core module 7. Aims All BEng and MEng Chemical Engineering Degrees This module is aimed at applying knowledge of chemical reaction rates to the sizing and operation of chemical reactors. Students apply stoichiometry, reaction kinetics and chemical equilibrium to different types of chemical reactions. They also develop an understanding of how a chemical reactor would work or is working, learn to apply the methods for sizing ‘ideal’ reactors for different reaction types, and achieve and understanding of the basics of ‘non-ideal’ flow and their application in sizing of ‘nonideal’ reactors. Learning materials are supported by practical laboratory experiments; e.g. measuring residence time distributions, determining rate constants. 8. Syllabus Main objectives in reactor design. A review of stoichiometry, reaction kinetics and chemical equilibrium. Batch reactors: design equation. Integral and differential techniques for investigating kinetics. Batch reactor sizing. Continuous reactors: Design equations for plug flow and continuous stirred tank reactors and their analytical and numerical solution. Reactor combinations. Autocatalytic reactions. Multiphase reactions: product distribution; selectivity and yield. Rate equations for multiple reactions. Parallel and series reactions and optimum choice of reactor type and operating conditions. Non-ideal flow: residence time and cumulative residence time distribution. Stimulus response techniques. Mean residence time. Non-ideal flow models for estimating conversions; Segregated flow, tanks-in-series and axial dispersion models. Form 20 Version 3.0 (2007/2008) Heriot-Watt University - Module Descriptor Template (RAY) Module Title Chemical Reaction Engineering School Engineering and Physical Sciences Module Co-ordinator 3rd Year Director of Studies SCQF Level 9 Module Code Semester B49CC 1 On or OffCampus ON Credits 15 9. Learning Outcomes (HWU Core Skills: Employability and Professional Career Readiness) Subject Mastery Understanding, Knowledge and Cognitive Skills Scholarship, Enquiry and Research (Research-Informed Learning) Students will be able to: Understand the principles of batch and continuous reactors. Apply chemical principles of stoichiometry, reaction kinetics and chemical equilibrium to reactor design. Be able to size a chemical reactor for different types of reactions in both ideal and non-ideal flows. Apply methods to determine the size of chemical reactors. Recognise the limitations imposed by non-ideal flow. Personal Abilities Industrial, Commercial & Professional Practice Autonomy, Accountability & Working with Others Students will be able to: Apply mathematical techniques to analyse chemical reactions. Describe the features of batch and continuous reactors. Demonstrate competence in practical application of theory. Work in small groups on specific technical topics. Analyse and report on technical issues. 10. Assessment Methods Method Examination Communication, Numeracy & ICT 11. Re-assessment Methods Duration of Exam (if applicable) 2 hours Coursework Weighting (%) Synoptic modules? 70% B49CF Method Duration of Exam (if applicable) Examination (synoptic with B49CF) 3 hours 30% 12. Date and Version Date of Proposal 29 Feb 2008 Date of Approval by School Committee Date of Implementation Version Number 1.0