Elements Found in the Human Body
advertisement

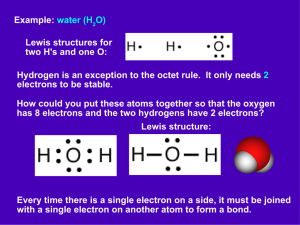
Chemical Bonding Worksheet Honors Name______________________ Period Date Draw the following covalent bonds using Lewis Structures. 1. 2 Hydrogen atoms 5. 1 carbon and 2 Oxygen atoms 2. 2 Fluorine atoms 6. 2 hydrogen and 1 Oxygen atoms 3. 2 Oxygen atoms 7. 1 Hydrogen and 1 Chlorine atom 4. 2 Nitrogen atoms 8. 1 Phosphorus and 1 Nitrogen atom The following atom will form ionic bonds. Draw the ionic bonds using Lewis structures. 9. Sodium and Chlorine 12. Strontium and Sulfur 10. Potassium and Bromine 13. Beryllium and Selenium 11. Magnesium and 2 Iodines 14. 2 Sodium and Sulfur 15. What are valence electrons? 16. According to the octet rule, how many valence electrons do atoms want? 17. Explain why atoms in the first group of the periodic table (Alkali Metals) are very reactive, but elements in the last group of the periodic table (Noble Gases) are not at all reactive. Reactive means that the element is likely to bond with other elements. (think about bonding, the octet rule, and valence electrons) 18. Draw the Lewis structure for Carbon below, next to it write how many bonds it can make. 21. Draw the Lewis structure for Hydrogen below, next to it write how many bonds it can make. 19. Draw the Lewis structure for Oxygen below, next to it write how many bonds it can make. 22. Draw the Lewis structure for Sulfur below, next to it write how many bonds it can make. 20. Draw the Lewis structure for Nitrogen below, next to it write how many bonds it can make. 23. Draw the Lewis structure for Neon below, next to it write how many bonds it can make. 24. How many valence electrons do the following atoms need to fulfill the octet rule? d. Neon? a. Hydrogen? e. Lithium? i. b. Strontium? c. Selenium? h. Gallium? f. Argon? Tin? g. Bromine? 25. Label the following as either an ionic (I) and or a covalent (C) bond. You will need to look up whether the elements involved are metals or nonmetals n. N2 a. CaCl2 o. NaI b. CO2 p. NO2 c. H2O q. Al2O3 d. BaCl2 r. FeCl3 e. O2 s. P2O5 f. NaF t. N2O3 g. NaS u. H2 h. S2 v. K2O i. SO3 w. KI j. LiBr x. P4 k. MgO y. CH4 l. C2H5OH z. NaCl m. HCl Use the following statistics on how many pounds of each element are in the human body to find the percentages of each element. Find the percentages by dividing the # pounds/ 150 pounds. Then, multiply the number by 100%. Then create a bar graph using the percentages. Element Oxygen Carbon Hydrogen Nitrogen Calcium Phosphorus Potassium Sulfur Sodium Chlorine Magnesium Iodine Iron # pounds 97 27 15 4.5 3 1.3 0.5 0.5 0.25 0.25 0.06 Trace Trace % of body Less than 0.01% Less than 0.01% Elements in Your Body 70 60 50 40 30 20 10 0 Elements Found in the Human Body The elements listed in the table below are found in your body. Fill in the information about each element using your Periodic Table of Elements. Round the atomic mass to the nearest whole number. Element Oxygen Carbon Hydrogen Nitrogen Calcium Phosphorus Potassium Sulfur Sodium Chlorine Magnesium Iodine Iron Symbol Atomic # Atomic Mass Protons Neutrons Electrons Bonding Basics - Covalent Bonds Complete the chart for each element. Element # of Protons Carbon Chlorine Hydrogen Phosphorus Oxygen Sulfur Nitrogen Complete each covalent bond. (1) Example of Lewis dot: Hydrogen + Hydrogen # of Electrons # of Valence Electrons # of Electrons to Fill Outer Shell (2) Hydrogen + Oxygen (3) Chlorine + Chlorine (4) Oxygen + Oxygen (5) Carbon + Oxygen (6) Carbon + Hydrogen