09. Weathering
advertisement

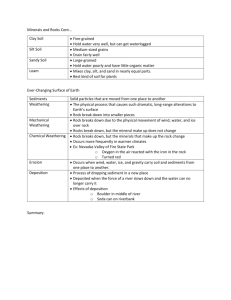
WEATHERING: Most rocks are unstable at the Earth's surface > they react with oxygen in the air, water, and organic matter > it is the external energy from the sub that drives the HYDROLOGICAL CYCLE, which creates the water flow, etc. leading to exposure of rocks to these agents > weathering is in interactive combination of MECHANICAL and CHEMICAL weathering processes (1) MECHANICAL WEATHERING: >the main role of mechanical weathering is to increase the surface area of the rock to make the chemical weathering processes more effective >> there are several forms of mechanical weathering: a. Unloading: >as rocks are brought to earth's surface by the erosion of overlying rocks, pressure is released and the rock expands by cracking to form JOINTS (a joint is a crack or break in a rock where there has been no movement of one block of rock past the other; if there has been movement, then it is called a fault) >Note: when a mine shaft is opened up underground, the release of pressure generates unloading stresses that will try and push the rocks into the shaft and close it up (this can happen catastrophically when there is a sudden rock burst). This leads to the need of providing supports and rock bolts to keep the rocks in place. b. Frost-Wedging: > when WATER freezes, there is a 9% increase in volume. Water in cracks of rocks, when it expands on freezing, exerts enormous pressure that can help break up a rock c. Biological Activity: > plant roots can push down and pry apart rocks >lichens and moss enhance breakdown of rock surfaces d. Heating-Cooling Cycles: > in deserts, where there is usually a large temperature variation between night and day, it is thought that the constant temperature cycling can help break rocks apart >> different minerals have different coefficients of thermal expansion, and the stresses this generates in the rock can break down the boundaries between different minerals e. "Bumping": > the constant bumping of rock fragments being carried in a stream, or the blasting of particles against rocks by wind, mechanically wears down rocks and rock fragments (2) CHEMICAL WEATHERING : Breaks down rock components and internal structures of minerals, converts original constitutents to new minerals or releases them into the environment. Of the two aspects of weathering (mechanical and chemical), chemical weathering is the most important. Only in very dry, cold climates (like part of the Arctic) does mechanical weathering exceed chemical weathering in importance. Let's look at the different chemical weathering processes: (a) Action of Water: > water at and near the earth's surface is acidified > it becomes acidified by contact with CO2 or S or interaction with the roots of plants or decaying organisms > what makes acids so powerful at weathering minerals is the hydrogen ion (H+ ), which is small and charged and can worm its way into minerals and break bonds > the most important of these acids is carbonic acid, because all rainwater interacts with CO2 in the atmosphere to become weakly acidic CO2 + H2O <> H2 CO3 >then some of the acid dissociates : H2 CO3 <> H+ + CO3 Let's look at the effect of acidic water on the chemical weathering of specific materials: LIMESTONE weathering: > CaCO3 (calcite) + 2H+ > H2 O + Ca2+ + CO2 >> the Ca ions are carried away in solution, and the carbon dioxide bubbles off (This is why calcite bubbles when you add weak Hcl to test for it) >> hence, the effect of chemical weathering of limestone is to completely dissolve it, so there is nothing left >>> it is this complete dissolution that leads to the formation of underground caves and channels. If there is collapse of the overlying rocks and soils into these caves, a very irregular ground surface can result. This is called karst topography. FELDSPAR weathering: e.g. 2KAlSi3O8 (K-feldspar)+ 2H+ + H2O > 2K+ + Al2Si2O5(OH)4 (clay) + 4SiO2 (in solution) > the acid breaks apart the framework structure of the feldspar, leaching out K and some of the Si, leaving behind a solid material, clay, with a sheet structure >since feldspar is the most abundant mineral in the earth's crust, it is not surprising that clay is the most abundant solid by-product of the chemical weathering process (b) Oxidation: where an element loses electrons > oxygen from the atmosphere is important in the chemical weathering of iron-bearing minerals, since Fe can occur in the ferrous (reduced) or ferric (oxidized) state e.g. 2Fe3 O4 (magnetite) + (1/2) O2 > 3Fe2O3 (hematite) e.g. 2Fe2SiO4 (olivine) + O2 + H2 O > 4FeO(OH) (limonite) + 2SiO2 (in solution) (Note: it is the oxidized iron oxides, like limonite, that give soils a reddish colour) (Note: an intermediate step in the oxidation of Fe-bearing silicates is the formation of an Fe-bearing clay, with subsequent breakdown to an oxide) (Note: oxidation of sulfide minerals, such as pyrite, can lead to the formation of sulfuric acid, which is a much stronger acid than carbonic acid. This is a problem where there are sulfide mines (e.g. mining of chalcopyrite) that results in the digging up of large amounts of sulfides. In the waste dumps of such mines (called the tailings dump), sulfides exposed to the atmospher are oxidized, and the S can combine with water to form sulfuric acid. These acid-rich waters can then flow off into local streams and lakes. This problem is called the problem of ACID MINE-TAILINGS. Furthermore, the smelting of the metalrich sulfides to extract the metals can then lead to SO2 being pumped up smoke stacks into the atmosphere, where it combines with water to form sulfuric acid, which leads to human-produced ACID RAIN. The reaction for the oxidation of pyrite is: FeS2 (pyrite) + O2 + H2O > Fe2O3 + H2SO4 (sulfuric acid) Consider the Chemical Weathering of a Granite: > 70% of the granite is feldspar >> it weathers to solid clay particles, with K and some Si going into solution > 10% of the granite is biotite mica (and/or amphibole) >> it weathers to solid limonite, and Si going into solution > 20% of the granite is quartz >> it is virtually unaffected by the weathering, forming sand-sized quartz grains as the rest of the rock crumbles around it Controls on the Rate of Weathering: (1) CLIMATE is the main factor controlling the weathering rate > the rate is higher when the following three are higher: a. rainfall, b. temperature, and c. biological activity (2) PARENT MATERIAL is a secondary factor controlling weathering rate a) Mineralogy: the Goldich Stability Sequence shows the relative resistance of different minerals to the weathering process > the least resistant mineral is calcite > the order of increasing resistance to weathering of the remaining common minerals is simply the Bowen's Reaction Series >> the higher up the Bowen Reaction Series, the less resistant the mineral (e.g., olivine is the least resistant common silicate mineral) >> the lower down the BRS, the more resistant the mineral (quartz, very low on the series, is the most resistant mineral on the BRS) >>> the most stable end products of the weathering process are the four minerals at the bottom of the Goldich Stability Sequence: Quartz, Clay, Hematite, Limonite. Economic Importance of Weathering: (1) forms aggregates that we use for engineering purposes (e.g., road and building construction) (2) forms soil > soils are valuable: (a) because we grow our crops in soil, and (b) there are some economic ore deposits related to the weathering process (e.g., bauxite ore deposits that we mine for aluminum) Let's talk about SOILS: > a soil forms over time by the chemical transformation of aggregate >> it develops by the drip action of water flowing through it to produce a soil >> a typical soil, once developed, contains about 25% air, 25% water, 45% mineral matter (clay, limonite, etc., etc.), and 5% dead organic matter (called HUMUS) > the drip action is like a drip coffee maker, with the ultimate formation of layers of different character (yielding a SOIL PROFILE) >> the upper layer, called the A Horizon (or Zone of Leaching), is where the water LEACHES (i.e., extracts by dissolving) ions out and carries them downwards; tiny particles of clay and other materials can also be carried down (Note: at the top of the A Horizon, there is a layer rich in organic material and the roots of plants. It is called the O Horizon.) >> in the B Horizon (or Zone of Accumulation), some of the ions carried down are precipitated, and some of the solid particles accumulate >> beneath the B Horizon is the C Horizon, which is basically just the broken surface of the solid rock beneath There are 3 main types of Soil Profiles: the type that forms depends on the climate Type 1: PEDOCAL > this type of soil forms in an arid climate >> because of the low rainfall, not much leaching happens and the A horizon in thin; because there is too little rainfall for good plant growth, the O horizon is thin >> only very soluble ions (such as Ca) get carried down, and they can be readily precipitated in the B horizon (there is not enough water flow to carry them out of the system); this can lead to the formation of hard nodules of CaCO3 (i.e., calcite) in the B Horizon. If a thick, hard layer of these forms, it makes for a rock-like layer called a caliche. > a pedocal soil is VERY POOR FOR AGRICULTURE!! (no good nutrients, rock-hard in places, no humus-rich part, low rainfall) >> most of western USA is pedocal soil (but this can of soil can be made to yield crops by massive irrigation, which is what happens in much of California Type 2: PEDALFER >this type of soil forms in a temperate climate (reasonably warm; reasonable rainfall) >> the Kingston area, and much of eastern USA and Canada, has this kind of soil >> the good rain and warmth allows for a thick A horizon with lots of humus >> the B horizon is thick with lots of clay and quartz, and some oxides of Al and Fe > a pedalfer soil is GREAT FOR AGRICULTURE!! Type 3: LATERITE > this type of soil forms in a humid tropical climate (like a rain-forest) >> these form in equatorial regions of the world >>it has a thin A horizon, and there is little humus, because the very warm, abundant rain leaches out all the nutrients >> although there can be lots of plant growth, it is living only in the thin outer part of the soil; as plants die, they rot rapidly, and the nutrients are immediately sucked up by the still-living plants >> since there is abundant leaching with the warm, plentiful rainwater, the B horizon (zone of accumulation) is very thick >> the leaching action is so extensive that even the clay minerals get completely broken down, with all the Si leached away, leaving behind abundant Fe- and Al-hydroxides in the B horizon > a laterite is POOR FOR AGRICULTURE!! >> this seems counter-intuitive, since there is warmth and lots of rain. However, the soil is really nutrient-poor. When rainforest is cleared for agriculture, you get good crops for a year or two until all the nutrients are used up, and then you have to move on and clear more rainforest and start over again. > laterites are the main source of aluminum metal >> if a laterite soil forms over a felsic rock, rich in Al, then an ore deposit might form >> the extensive leaching of the feldspars in the granite leads to the breakdown into first clays and then Al-rich oxides >>> the mineral BAUXITE (Al2O3. 3H2O) is what we mine from these soils >>> bauxites are great sources of Al because: a.) there is a high concentration of Al (a granite or syenite starts with 8% Al, whereas the laterite soil (i.e., bauxite) has up to 55% Al) b.) the Al is in a non-silicate mineral (bauxite) compared to the silicate feldspar, and hence it takes less energy to smelt and isolate the Al c.) the ore is in the form of a soil at the surface of the earth, which makes it easy to dig out >>> syenite is better than granite for producing a bauxite ore, because the syenite is just a granite without quartz, and quartz, because it doesn't break down readily during weathering, is a contaminant in the ore that you don't want; hence, syenite is better Last revision: 6 November 2000 These pages and their contents are Copyright © of the Department of Geological Sciences, Queen's University 20002001 (except as noted). If you have any problems with this web service, Email badham@geol.queensu.ca Back to APSC151 Course Notes Page