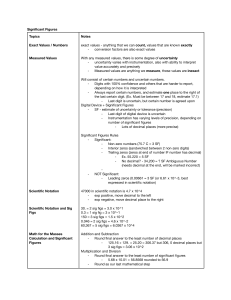
Genera Rules for Metrics

Metrics and Significant Figures – Notes
1) Prefixes/amounts to know : Prefix
Mega
Kilo
Deka
Deci
Centi
Micro
Nano
Base unit
Milli
Pico
Amount
10 6
10 3
10 1
10 0
10 -1
10 -2
10 -3
10 -6
10 -9
10 -12
* - you are responsible for these prefixes, powers, and symbols
2) Base and Derived units to know: Name Symbol
Gram
Meter
Joule
Liter
Celsius
Kelvin
Mole g m
J o
L
C
K mol
Name___________________________ mass
Symbol
M k * da any metric unit * d c m
* n p
*
*
*
*
*
Use linear distance energy volume temperature temperature amount of substance
Second s
Squared meter m 2
Cubic meter m 3
Mass per unit g/L volume amount per unit mol/L volume time surface area volume density molarity
3) Significant figures are a way of being exact while measuring
A) The amount of significant figures relies on the accuracy of the measuring device
1) accuracy (close to true value)
2) precision (how close repeat measures are to each other)
3) human error may play a role, so need to be careful when measuring i.e parallax (error in measurement due to the position of the observer)
B) To record significant figures when measuring: record the measurement to the smallest degree that is available (i.e. cm on a meter stick) and then estimate one more place
C) Rules of significant figures for given individual numbers:
1) All non-zero numbers are significant
2) All zeros between non-zero numbers are significant
3) All zeros to the right of the decimal and to the right of the non-zero numbers are significant
4) All zeros used for spacing the decimal point are not significant.
They are just placeholders.
5) Special cases: a.
A line may be placed over a zero to show that it is the last significant figure. b.
A decimal at the end of a number shows that all of the numbers before the decimal are significant figures c.
Only measurements have significant figures as counted numbers are called exact numbers. d.
Counting numbers and defined numbers have an unlimited number of significant figures.
D) Rules for Sig. figs in combination with mathematical processes
1) when adding or subtracting, the answer may only contain as
many decimal places as the measurement having the least
amount of decimal places
2) when multiplying and dividing, the answer may only contain
as many sig figs as the measurement having the least number
of sig figs
4) Additional information:
A) Percent error is a way of quantifying how far a measurement deviates from the true or actual value
General equation: (Actual value - Experimental value) x 100 = % error
Actual value
B) Percent yield is a way of quantifying how much measured (experimental) in comparison to how much you should have measured (actual)!
General Equation: (Experimental value) x 100 = % yield
Actual value