Glycolysis
advertisement
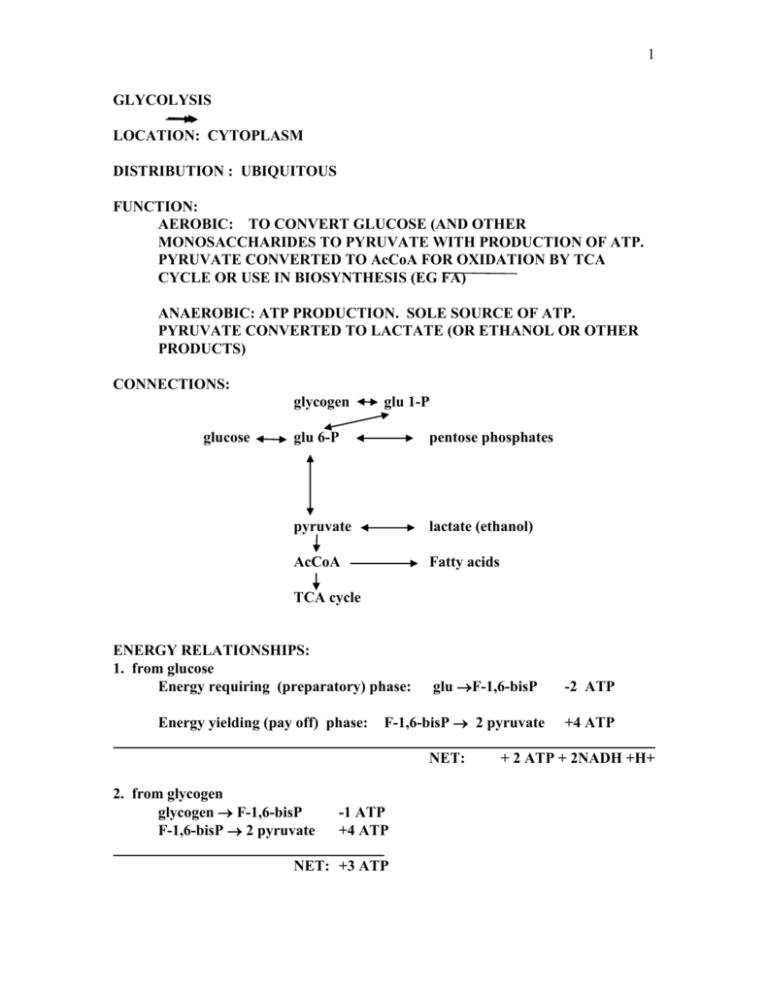
1 GLYCOLYSIS LOCATION: CYTOPLASM DISTRIBUTION : UBIQUITOUS FUNCTION: AEROBIC: TO CONVERT GLUCOSE (AND OTHER MONOSACCHARIDES TO PYRUVATE WITH PRODUCTION OF ATP. PYRUVATE CONVERTED TO AcCoA FOR OXIDATION BY TCA CYCLE OR USE IN BIOSYNTHESIS (EG FA) ANAEROBIC: ATP PRODUCTION. SOLE SOURCE OF ATP. PYRUVATE CONVERTED TO LACTATE (OR ETHANOL OR OTHER PRODUCTS) CONNECTIONS: glycogen glucose glu 1-P glu 6-P pentose phosphates pyruvate lactate (ethanol) AcCoA Fatty acids TCA cycle ENERGY RELATIONSHIPS: 1. from glucose Energy requiring (preparatory) phase: Energy yielding (pay off) phase: glu F-1,6-bisP F-1,6-bisP 2 pyruvate NET: 2. from glycogen glycogen F-1,6-bisP F-1,6-bisP 2 pyruvate -1 ATP +4 ATP NET: +3 ATP -2 ATP +4 ATP + 2 ATP + 2NADH +H+ 2 glucose 2 ADP 2 lactic acid 2 ATP 123kJ/mol 60 kJ = 49% yield glucose 6 CO2 2828kJ/mol (Lactic yields only 2% of total available energy) PATHWAY SHOW OVERHEAD OF PATHWAY KINASES, ISOMERASES, MUTASES, 1. TRIOSE PHOSPHATE ISOMERASE: REQUIRED IN ORDER THAT ALL OF GLUCOSE CAN BE CONVERTED TO PYRUVATE. NOTE SOURCE OF CARBON ATOMS NOTE: NO NET OXIDATION, INTERNAL REARRANGEMENT 2. WHY LACTIC ACID UNDER ANAEROBIC CONDITIONS? REGENERATION OF NAD. UNDER AEROBIC CONDITIONS THIS ACHIEVED BY MITO SHUTTLE SYSTEMS 3. PHOSPHORYLATION OF GLUCOSE Traps glucose in cell, creates more reactive compound Hexokinase: relatively non-specific-acts on glu, mann, fruc, Km in low uM range, bld glu 4-8 mM therefore active most of the time, occurs in muscle and brain. Inhibited by G-6-P Glucokinase: (hexokinase IV, an isozyme) predominates in liver. Km 5 - 10 mM, therefore only active at high concentrations of glucose, activity changes as [glu] changes. Not inhibited by G-6-P. Fructokinase: (liver) fructose F-1-P DHAP + glyceraldehyde G-3-P Note: liver has high concentration of fructokinase GLUCOSE TRANSPORTERS: Passive transport, intracellular conc lower than serum conc. GLUT 1-7 12 TM domain proteins Types 1 and 3 present in all cells have Km for glu of about 1 mM. Therefore transport glucose at all times at a constant rate 3 Type 2 present primarily in liver, Km is ~60 mM (Voet and Voet), therefore responds essentially linearly with changing bld glucose. It is never saturated and therefore never limits the rate of glucose entry. Type 4: muscle and fat cells: present in intracellualr vesiclaes which fuse with membrane in of presence of insulin, thereby increasing number of transport molecules in cell surface membrane. 4. LACTATE DEHYDROGENASE: EXAMPLE OF ISOZYME: heart and muscle (liver) form Isozyme: different proteins from the same species which catalyse the same reaction LDH ISOZYMES TETRAMERIC ENZYME OF VARIOUS COMBINATIONS OF A AND B SUBUNITS A SUBUNIT (M) HIGH IN LIVER AND MUSCLE B SUBUNIT (H) HIGH IN HEART A4 WORKS BEST IN DIRECTION TOWARDS LACTIC PRODUCTION AND REGENERATION OF NAD. EG DURING ACTIVE ANAEROBIC GLYCOLYSIS IN MUSCLE B4 HEART USES LACTATE AS A FUEL AND CONVERTS IT TO PYRUVATE. THIS FORM IS INHIBITED BY PYRUVATE ENSURING THAT EXCESS PYRUVATE WILL NOT BE WASTED BY CONVERSION TO LACTATE. 5. ENERGY CONSERVATION: substrate level phosphorylation 1. glyceraldehyde 3 P dehydrogenase I. oxidation of CHO COO II. phosphorylation of COO energy of oxidation stored in thioester and used to drive phosphorylation reaction see overhead NOTE: arsenate replacec phosphate in G3P dehydogenase but then spontaneously yields 3Pglycerate with no ATP production. AsO3-2 4 WHAT IS ENERGY YIELD IN PRESENCE OF ARSNATE? REGULATION: allosteric phosphorylation 1. HEXOKINASE Inhibited by G-6-P which accumulated if other reactions are inhibited. 2. Pyruvate kinase 4 isoenzymic forms inhibited by ATP, activated by F-1,6-Bis P see fig 12.17 Horton liver form also inhibited by phosphorylation 3. PHOSPHOFRUCTOKINASE Main point of regulation ATP, citrate AMP, F-2,6-bisP inhibited by H+ ions: eg if lactic acid is not cleared pH drops and activity of PFK1 slows Sensitive to energy charge of the cell. NOTE: [ATP} remains relatively constant but large changes in AMP and AP occur because they are present in lower concentration. Thus a small decrease in [ATP] results in large change in AMP ATP ADP 2 ADP ATP + AMP FRUCTOSE-2,6-BISP Discovered in 1980 Potent activator of PFK, effective in uM range F-6-P F-2,6-bisP PFK-2/fructose-2,6-bisphosphatase (single enzyme in liver) PFK-2 is inhibited by citrate and by phosphorylation whereas F26bPase is stimulated by phosphorylation SUMMARY ALLOSTERIC REGULATION hexokinase G6P PFK ATP, citrate AMP, F26bisP PFK-2 F26bisPase citrate, phosphorylation phosphorylation 5 pyruvate kinase F-16-bisP phosphorylation PHOSPHORYLATION pyr kinase PFK-2 decreases F2,6 bis Pase (liver) result [F2,6,bisP] drops and activity of PFK1 Phosphorylation results in PK activity, a drop in F-2,6 bisPase, and a PFKase Therefore phosphorylation results in inhibition of glycolysis in liver Phosphorylation increases when bld glucose levels are low in response to the presence of the hormone glucagon in the blood. PASTEUR EFFECT Inhibition of glucose consumption by oxygen