file - BioMed Central
advertisement

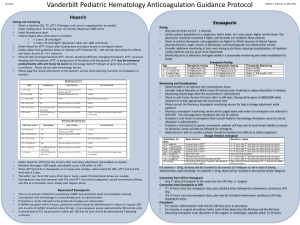
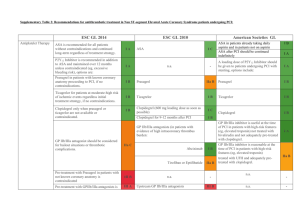
Additional material Section C: Data extraction tables for clinical trials Summary of small trial: Data extraction and appraisal Reference Intervention Subjects Outcome measures Kuch et al Aspirin (150 mg daily) and n=201 1. Myocardial infarction. (1995)[5] 1. UFH (intra-venous UFH/aspirin 67 2. Recurrent ischaemia, ECG. RCT bolus 5000 IU and Enoxaparin/aspirin 67 3. Recurrent angina (pain). One centre infusion 800-1000 U/hr). Aspirin alone 67. 4. Worsening congestive heart failure or and design (Poland) 2. Enoxaparin (40 mg sub- pulmonary oedema. cutaneous). Consecutive patients 5. Ventricular fibrillation or tachycardia. Aspirin alone. admitted to coronary care 6. Deaths (30-day). Treatment: Planned and unit with unstable angina and 7. Total recurrent ischaemic events mean treatment duration ST- depression. unknown. Median duration of Mean age 54 7 years. 8. Total cardiac events. hospitalisation approximately 84% males. 9. Total major complications (strokes and 3. 17 days in each group. (ECG/pain). bleeding). Follow-up: Up to 30 days. Results: None were statistically significant. All presented as UFH, enoxaparin and aspirin 1. Myocardial infarction: 11.9%, 13.4% and 10.4%. 2. Recurrent ischaemia (ECG): 11.9%, 11.9% and 14.9%. 3. Recurrent angina attacks (pain): 8.9%, 11.9% and 14.9%. 4. Worsening of congestive heart failure or pulmonary oedema: 7.5%, 8.9% and 11.9%. 5. Ventricular fibrillation or tachycardia: 1.5%, 3.0% and 3.0%. 6. Deaths (30-day): None in any group. 7. Total recurrent ischaemic events (ECG and anginal pain): 20.9%, 23.9% and 31.3%. 8. Total cardiac events: 41.8%, 49.2% and 56.7%. 9. Total major complications (strokes and bleeding): 3.0%, 0 and 0. Comments Currently only available as an abstract. Sampling/recruitment: Insufficient detail about inclusion/exclusion criteria, and similarity between groups unknown. The setting (Coronary Care Unit) and level of patient monitoring may not reflect those typical in the UK. Treatment regime unclear, eg frequency of enoxaparin administration, target activate partial thromboplastin time (for UFH) and treatment duration not specified. 1 Results: Unclear when outcomes were measured. P values and confidence intervals not presented. Unclear whether authors performed intention-to-treat analysis. Jadad score[1] 1/5: Randomised (1 + 0); Double blind (0 + 0); Withdrawals and dropouts described (0). Method of randomisation not described, blinding status and completeness of follow-up unknown. Notes: UFH = unfractionated heparin 2 Summary of ESSENCE trial: Data extraction and appraisal Ref and Intervention Subjects Outcome measures 1 mg/kg enoxaparin sub- n=3171 Primary (triple composite) endpoint (at 14 1997 cutaneous 12 hourly and UFH 1564, enoxaparin 1607 days): ESSENCE placebo (bolus and infusion); Recent onset angina at rest 1. Death (any cause or cardiac arrest) Or sub-cutaneous placebo 10 minutes within 24 hours 2. Myocardial infarction (or re-infarction) Double-blind injections and UFH intra- of randomisation. Additional 3. Recurrence of angina. RCT venous bolus (usually 5000 evidence of underlying Secondary outcomes: 176 centres U) followed by infusion (dose ischaemic heart disease. 1. 10 countries adjusted according to in US, activate partial Median ages UFH and Canada, thromboplastin time, typically enoxaparin 64 and 65 years myocardial infarction) at 48 hours, 14 South 55-85 secs.). respectively (inter quartile and 30 days. America and All patients received aspirin range not stated). Mean 3. Europe. 100-325 mg daily. ages UFH and enoxaparin Length of follow-up 30 days, extended to one Patient Treatment: Minimum 48 64 and 63 respectively. year (median 17.3 months). enrolment hours, maximum 8 days Approximately 66% males in Oct 1994 – (discontinued at hospital each group. May 1996. discharge, new myocardial design Cohen et al. [2,4,6-8] Triple composite endpoint at 48 hours and at 30 days 2. Double composite endpoint (death/ Major or minor haemorrhage. infarction, revascularisation or death). Median treatment 2.6 days (both groups), range 2-8 days. Results (RRR (relative risk reduction); OR (odds ratio) 95% confidence intervals) Primary triple composite endpoint at 14 days: UFH 19.8% cf. enoxaparin 16.6% (RRR 16.2%; OR 0.80 0.67 to 0.96; P=0.02). Secondary outcomes: 1. Triple composite endpoint: At 48 hours (UFH 7.4% cf. enoxaparin 6.2% (RRR 16.2%;OR 0.83 0.62 to 1.09; P=0.16); at 30 days UFH 23.3% cf. enoxaparin 19.8% (RRR 15.0%;OR 0.81 0.68 to 0.96; P=0.02); at one year UFH 34.7% cf. enoxaparin 31.0% (RRR 10.7%;OR not stated; P=0.02) 2. Double composite endpoint (death/myocardial infarction): No significant difference at 48 hours, 14 or 30 days. Eg at 48 hours UFH 1.3% cf. enoxaparin 1.1% (RRR 16.6%; OR 1.20 0.64 to 2.28; P=0.57); at one year UFH 13.1% cf. enoxaparin 11.1% (RRR 15.5%; OR not stated; P=0.08). 3. Major or minor haemorrhage at 30 days in patients receiving at least one dose of study medication[6]: Major bleeding UFH 7.0% cf. enoxaparin 6.5% (P=0.57), the majority during/after coronary artery bypass grafting following 3 discontinuation of study medication. Minor bleeding UFH 8.8% cf. enoxaparin 13.8% (P<0.001), most frequently injection site ecchymosis. No cases of heparin induced thrombocytopenia in either group. 4. Individual endpoints: Death, myocardial infarction and recurrent angina presented at 30 days and one year used for modelling, but not presented here. Revascularisations: At 30 days: All revascularisations: UFH 32.2% cf. enoxaparin 27.0% (RRR 16.2%; P=0.001); coronary artery bypass grafting: UFH 13.7% cf. enoxaparin 12.3% (RRR 10.0%; P=0.25); percutaneous transluminal coronary angioplasty: UFH 18.7% cf. enoxaparin 14.7% (RRR 21.6%; P=0.002). At one year: All revascularisations: UFH 39.8% cf. enoxaparin 34.8% (RRR 12.7%; P=0.0.002); coronary artery bypass grafting: UFH 19.3% cf. enoxaparin 17.9% (RRR 7.2%; P=0.26); percutaneous transluminal coronary angioplasty: UFH 22.1% cf. enoxaparin 17.9% (RRR 19.0%; P=0.004). Sub-groups analyses on the triple endpoint at 14 days suggested a significant advantage of enoxaparin over UFH for the following patient sub-groups: 65 years old; ECG changes; ST depression; prior percutaneous transluminal coronary angioplasty; prior aspirin use; former or non-smoker. Comments Sampling/recruitment: Inclusion/exclusion criteria explicit. Some indication of sample size calculations in separate publication[8]. Country adjusted odds ratios not significantly different from unadjusted odds ratios. Description of patients’ characteristics allows comparison with local settings. Method of randomisation not described in paper, however, information from pharmaceutical company suggested the randomisation was via sequence generation by the Biometrics department of the company. Follow-up appeared to be complete. Blinding appeared to be maintained. Groups had similar baseline characteristics and appeared to be treated similarly during trial. Results: intention-to-treat analysis for all efficacy endpoints. confidence intervals and P values presented for the majority of outcomes. Per protocol analyses for triple endpoint (48 hours, 14 and 30 days) were comparable with that of the intentionto-treat analysis. Only per protocol analysis presented for adverse effects (however, in this case it did not alter the results) and confidence intervals not presented, only P values. Sub-group analyses should be interpreted with caution since these are likely to yield spurious results through chance alone. Potential confounding factors: Variation in aspirin dose (100-325 mg daily). At 6 to <12 hours, activate partial thromboplastin time was within the therapeutic range (55-85 sec) for only 30% of patients receiving UFH. activate partial thromboplastin time within therapeutic range for 46-60% of patients (at 12-96 hours); under range in 15-18% patients (up to 96 hours) and over range in 23-52% patients. However, such levels may be better than those achieved in routine practice. Jadad score 5/5: Randomised (1 + 1); Double blind (1 + 1); Withdrawals and dropouts described (1). Notes: UFH = unfractionated heparin 4 Short and long-term treatment phases for TIMI 11B trial Phase and treatment Enoxaparin UFH (minimum 3 days) Short-term 1 bolus 30 mg enoxaparin 1 placebo bolus intra-venous bolus and 1 placebo bolus and 1 bolus 70 U/kg UFH intra-venous infusion placebo 15 U/kg/h UFH (activate partial thromboplastin time 1.5-2x control) sub-cutaneous injection 1 mg/kg enoxaparin 12 hourly placebo Long-term. From first of hospital enoxaparin sub-cutaneous injection 12 hourly placebo sub-cutaneous injections discharge or day 8. Continued for 60 mg if patient 65 kg 35 days. 40 mg if patient <65 kg Summary of TIMI 11B trial: Data extraction and appraisal Ref and Intervention Subjects Outcome measures Antman et al. Intervention see table n=3910 Primary (triple composite) endpoint: 1999[3] above. Short-term phase: UFH 1957, enoxaparin 1953 1. All cause mortality TIMI 11B UFH cf. enoxaparin. Long- People with ischaemic 2. Recurrent myocardial infarction Double-blind term phase: Enoxaparin cf. discomfort 5 minutes 3. Urgent revascularisation RCT placebo for up to 35 days duration at rest within 24 Secondary endpoints: 200 centres after discharge. hours of randomisation with 1. Individual endpoints. 10 countries All patients received aspirin evidence of ischaemic heart 2. Death/non-fatal myocardial infarction. (in North and (100-325 mg/day). disease. 3. Haemorrhage. South Treatment duration: Short- At initial randomisation Endpoints: 8 days (short-term), 43 days America and term phase, median (inter median age 66 and 65 years (long-term), 48 hours and 14 days. Europe). quartile range): UFH 3.0 for UFH and enoxaparin Enrolment (2.99, 3.98) days cf. respectively (inter quartile 1996-1998 enoxaparin 4.6 (2.97, 6.59) range 57, 72 and 56, 73). days. Long-term phase Approximately 65% males. design Follow-up 43 days. mean/median unknown. Results: Short-term phase (at 8 days). Primary endpoint: UFH 14.5% cf. enoxaparin 12.4% (RRR 14.6%; OR 0.83, confidence intervals0.69 to 1.00; P=0.048). Individual endpoints and death/myocardial infarction composite were all reduced in the enoxaparin group, although only significant for myocardial infarction rates (RRR 28.9%; OR 0.70, confidence intervals0.51 to 0.97; P=0.028). Long-term phase (at 43 days). Primary endpoint: UFH 19.7% cf. enoxaparin 17.3% (RRR 12.3%; OR 0.85, confidence intervals0.72 to 1.00; P=0.048). Individual endpoints and composite of death/myocardial infarction were all lower in the 5 enoxaparin group, but only reduction in urgent revascularisation was statistically significant (RRR 15.6%; OR 0.82, confidence intervals0.67 to 1.00; P=0.05). For patients without an endpoint by day 8 there was no significant difference in events to day 43. Other time points. At 48 hours: Triple composite: UFH 7.3% cf. enoxaparin 5.5% (OR 0.75, confidence intervals0.58 to 0.97; P=0.026). Secondary endpoints: myocardial infarction, urgent revascularisation and the composite of death/myocardial infarction all showed a reduction in events in the enoxaparin group, but none were statistically significant. The death rate was greater, though not statistically significant, in the enoxaparin compared with UFH group (11 cf. 6 deaths; OR 1.84, confidence intervals0.69 to 4.99; P=0.219), but this was reversed by day 8 (again, not statistically significant). At 14 days. Triple composite: UFH 16.7% cf. enoxaparin 14.2% (OR 0.82, confidence intervals0.69 to 0.98; P=0.029). Secondary endpoints: Individual endpoints and death/myocardial infarction composite all showed a reduction in events in the enoxaparin group, though none were statistically significant. Kaplan-Meier: The triple endpoint survival curves separated from 8 hours to day 14 and were then parallel to day 43 indicating no further relative treatment benefits from enoxaparin. Sub-group analyses on the triple endpoint at 14 days suggested a significant advantage of enoxaparin in some patient subgroups: T-wave inversion, ST depression, ECG changes and prior aspirin use. Adverse events (per protocol analysis): Major haemorrhage at 72 hours (during anticoagulation) and during initial hospitalisation, was slightly (not significantly) lower in the UFH cf. enoxaparin group, (0.7 cf. 0.8% and 1.0 cf. 1.5%). Minor haemorrhage at 72 hours and during initial hospitalisation, was significantly lower in the UFH cf. enoxaparin group (2.3% cf. 5.1% and 2.5% cf. 9.1% P<0.001 for both), with the majority of cases related to injection/cardiac catheterisation sites. Other adverse events, eg thrombocytopenia, not significantly different. Comments Sampling/recruitment: Indication given of sample size calculations. Description of patients’ characteristics enables local comparisons. However, inclusion criteria changed after 10 months to higher risk patients (after recruitment of approx. half the patients), following a blinded review by the Operations Committee, contradicting the statement that no interim analyses were conducted. Groups had similar baseline characteristics; however, the UFH group had a shorter treatment duration (due to the protocol design), median 3 cf. 4.6 days for enoxaparin group. Results: intention-to-treat analysis for all efficacy endpoints with presentation of confidence intervals and P values. Per protocol analysis for adverse effects with P values (not confidence intervals), however, intention-to-treat analysis would not have altered the results. Caution required regarding sub-group analyses that may yield spurious results through chance alone. Potential confounding factors: Variation in aspirin dose (100-325 mg daily). Jadad score 3/5: Randomised (method not described) (1 + 0); Double-blinding appeared to be maintained (1 + 1); Withdrawals (0, only main reasons given leaving 685 patients unaccounted-for. Unclear what effect this had on the ‘intention-to-treat’ analysis at 43 days). Notes: UFH = unfractionated heparin 6 Section C: References 1. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ et al.: Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996, 17: 1-12. 2. Cohen M, Demers C, Gurfinkel EP, Turpie AG, Fromell GJ, Goodman S et al.: A comparison of low-molecular-weight heparin with unfractionated heparin for unstable coronary artery disease. Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events Study Group. N Engl J Med 1997, 337: 447-452. 3. Antman EM, McCabe CH, Gurfinkel EP, Turpie AGG, Bernink PJLM, Salein D et al.: Enoxaparin prevents death and cardiac ischemic events in unstable angina/ non-Q-wave myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) 11B trial. Circulation 1999, 100: 1593-1601. 4. Fox K: The role of the antithrombins in improving outcome in unstable angina. Br J Cardiol 1998, 5: S7-S9. 5. Kuch M, Chmielewski M, Swiatowie A, Mamcarz A, Braksator W, Dhizniewski M et al.: Could heparin or enoxaparin added to aspirin alone therapy reduce cardiac events in patients suffering from unstable angina pectoris? - Study performed in 201 patients. Kardiologia Polska 1997, 47: I-140 (abstract 139). 6. Cohen M, Demers C, Gurfinkel EP, Turpie AG, Fromell GJ, Goodman S et al.: Low-molecularweight heparins in non-ST-segment elevation ischemia: the ESSENCE trial. Efficacy and Safety of Subcutaneous Enoxaparin versus intravenous unfractionated heparin, in non-Qwave Coronary Events. Am J Cardiol 1998, 82: 19L-24L. 7. Demers C: ESSENCE trial results: breaking new ground. Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q wave Coronary Events. Can J Cardiol 1998, 14: 15E19E. 7 8. Cohen M, Blaber R, Demers C, Gurfinkel EP, Langer A, Fromell G et al.: The ESSENCE trial: Efficacy and safety of subcutaneous enoxaparin in unstable angina and non-Q-wave MI: A double-blind, randomized, parallel-group, multicenter study comparing enoxaparin and intravenous unfractionated heparin: Methods and design. J Thromb Thrombolysis 1997, 4: 271-274. 8