X. Membership of the KVAST study group
advertisement

Sida 1 av 20
GASTROINTESTINAL PATHOLOGY PANCREAS and
PERI-AMPULLARY REGION
Recommendations from the KVAST Study Group of the Swedish Society for
Pathology, Autumn 2012
KVAST Study Group for hepatopancreatobiliary pathology:
Mikael Björnstedt (Stockholm)
Lennart Franzén (Stockholm)
Hans Glaumann (Stockholm)
Hans Nordlinder (Göteborg)
Richard Palmqvist (Umeå)
Pehr Rissler (Lund)
Åke Öst (Täby)
Caroline Verbeke (Stockholm)
These national guidelines provide recommendations for the handling, grossing and
microscopic reporting of pancreatic biopsies and surgical resection specimens. They include
a summary of the main morphological characteristics of common pancreatic tumours and
diseases. The recommendations are based on the experience of the study group, evidence
in the litteraturs, and recent classification systems.
2016-02-13
Caroline Verbeke
Sida 2 av 20
Table of contents
II. Key clinical information .................................................................................................................... 3
III. Instructions for specimen dissection (following formalin fixation) ............................................ 3
1. Specimen orientation and external inspection ..................................................................... 3
2. Identification and inking of the resection margins ............................................................... 3
3. Axial specimen slicing ............................................................................................................. 5
4. Inspection of the dissected specimen ................................................................................... 6
5. Tissue sampling ........................................................................................................................ 7
6. Lymph node sampling and allocation .................................................................................... 7
IV. Processing of pancreatic biopsies................................................................................................ 9
V. Information to be included in the pathology report ..................................................................... 9
1. Ductal adenocarcinoma and variants .................................................................................... 9
Table 5: Immunohistochemical profile of ductal adenocarcinoma of the pancreas (19) 11
2. Pancreatic intraepithelial neoplasia (PanIN) ...................................................................... 12
3. Intraductal papillary mucinous neoplasia (IPMN) .............................................................. 13
4. Mucinous cystic neoplasm (MCN) ....................................................................................... 14
5. Serous cystadenoma (SCA) ................................................................................................. 14
6. Solid-pseudopapillary tumour (SPT) ................................................................................... 15
7. Endocrine tumours and mixed adenoneuroendocrine carcinoma (MANEC) ................ 15
8. Acinar cell carcinoma............................................................................................................. 15
9. Chronic pancreatitis ............................................................................................................... 16
VI. Recommendations for reporting ................................................................................................. 16
VII. SNOMED-codes .......................................................................................................................... 16
VIII. References .................................................................................................................................. 17
IX. List of abbreviations ..................................................................................................................... 19
X. Membership of the KVAST study group ..................................................................................... 19
APPENDIX 1 ............................................................................................................................... 20
Sida 3 av 20
I. Instructions for specimen handling (prior to formalin fixation)
The surgical resection specimen should be sent unfixed to the pathology department for the
purpose of fresh tissue sampling for the biobank. Any structures or organs included in the
specimen in addition to the ones usually contained in the conventional surgical specimen
types (eg. SMV/PV resection, colon, …) may be suture marked by the surgeon. The
specimen should be opened along the greater curvature of the stomach and along the
duodenal wall opposite of the papilla of Vater, after careful blunt probing with a finger to
avoid cutting through possible lesions of the duodenum or around the papilla. If present, the
gall bladder should als be opened longitudinally. The lumina should be rinsed with water and
the specimen fixed free-floating for 24-48 hrs in plenty of 10 % neutral buffered formalin. It is
important that the specimen and in particular its surface remain intact. Therefore, the
specimen should not be opened in any other than the above-described way, and in
particular, the pancreatic and bile duct should not be probed or opened. One, maximal two
incisions in the axial plane can be made to allow tissue sampling for the biobank. Whether
fresh tissue samples can be taken (for biobanking or other purposes) is at the discretion of
the pathologist and should never jeopardise diagnostic reporting.
Trucut biopsies should be fixed immediately in 10 % neutral buffered formalin (4 %
formaldehyde).
II. Key clinical information
The referring clinician should satte the following important information on the clinical request
form: (1) the clinical diagnosis (suspected tumour entity, tumour localisation and cancer
origon) and/or (2) the indication for surgery or biopsy sampling, and (3) the specimen type
(Whipple's resection, pylorus-preserving pancreatoduodenectomy; total, distal or central
resection). Inclusion of additional organs or structures (eg. vein, mesocolon) should be stated
and the presence of possible marker sutures explained. It should be stated if and which preoperative treatment the patient received.
III. Instructions for specimen dissection (following formalin fixation)
1. Specimen orientation and external inspection
The specimen should be properly orientated and inspected. All key measurements should be
taken. For a Whipple's specimen this includes the length of the duodenum and distal
stomach (along the greater and lesser curvature), the length and maximum diameter of the
gall bladder, cystic duct, and (extrapancreatic) common bile duct. The pancreatic head
should be measured in 3 dimensions, ie. craniocaudal, mediolateral, anteroposterior. The
measurements of blood vessels, the spleen or any other organs or structures included in the
specimen should be taken. Any abnormalities identified on external specimen inspection
should be recorded. To allow optimal specimen dissection, surgical sutures and clips should
be carefully removed without disruption of the specimen surface, as this represents the
circumferential resection margin.
2. Identification and inking of the resection margins
A Whipple's resection specimen has 4 transection margins: the proximal (gastric) and distal
(duodenal) transection margins, which are of little if any clinical significance, and the
transection margins of the common bile duct and pancreatic neck. The latter margin is readily
identifiable, as it shows bare pancreatic parenchyma and contains the main pancreatic duct,
which in many cases is dilated and therefore easily seen. The extrapancreatic stump of the
common bile duct can be easily found by following the SMV groove (see below) 1-2 cm up
cranially.
Sida 4 av 20
The circumferential margins (figure 1) are easiest identified as follows:
- resection margin at the SMV groove ('SMV margin'): this surface is located immediately
below the pancreatic transection margin. It runs slightly obliquely along the medial aspect of
the pancreatic head. It normally has a smooth, slightly shiny surface and is often flanked on
either sides by multiple clips or sutures on small veins. If a venous resection was undertaken,
the venous segment will be found adherent to the SMV groove. It is recommended to ink the
resected vein with a different colour to facilitate identification of this tissue following specimen
slicing and during microscopic examination.
- resection margin facing the SMA ('SMA margin'): this surface lies to the left-posterior aspect
of the SMV groove, and in contrast to the latter, its surface is rough, fibrous and often
irregular. It is often wedge-shaped, ie. narrower towards the cranial aspect of the pancreatic
head, and broader towards the inferior pole
- Posterior resection margin: this is the fibrous but relatively smooth, flat surface at the back
of the pancreatic head, which extends from the SMA-facing surface to the posterior duodenal
wall
- Anterior pancreatic surface: this is not a resection margin but a free anatomical surface
facing the lesser sac. It extends from the SMV groove to the anterior duodenal wall. It is
usually smooth, but can on occasion be overlaid with adipose tissue. Tumour breaching of
this surface can be of prognostic significance and therefore the anterior surface should be
included in the assessment.
These surfaces should be inked according to an agreed colour code. Possible other
resection margins, eg. of a segment of vein, should be inked in a different colour and this
should be stated in the macroscopic description.
Amongst the circumferential resection margins the SMA facing margin is the only true
transection margin, ie. where the surgeon transsects tissue, in this case the soft tissue
adjacent to the SMA. The posterior margin and the margin at the SMV are so-called
dissection margins, where the surgeon bluntly dissects tissue along an anatomical plane. It
remains to be seen whether involvement of either type of margin is of prognostic significance
(1).
Figure
1.
Circumferential
resection
pancreatoduodenectomy specimen.
margins
and
surfaces
of
a
Orientation of distal pancreatectomy specimens is based on the position of the splenic artery,
which runs along the cranial aspect of the pancreatic body and tail. Margins in this specimen
type consist of the pancreatic transection margin, and the anterior and posterior surface of
the pancreatic body and tail, which should also be inked according to an agreed colour-code.
Sida 5 av 20
Resection specimens for chronic pancreatitis following the duodenum-sparing Beger
operation may consist of irregular tissue fragments, which are no further orientable and
whose entire surface can be inked with a single colour.
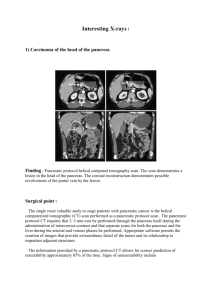
3. Axial specimen slicing
Various dissection techniques for pancreatoduodenectomy specimens are currently used
worldwide, including slicing along the plane of the pancreatic and bile duct, or so-called
bread slicing perpendicular to the longitudinal axis of the pancreatic body. Increasingly used
and recommended in these national guidelines is the axial slicing technique (figure 2), which
offers a number of important advantages (2). This method allows detailed assessment of the
local anatomy and direct comparison with findings on pre-operative scanning images. It is
easy to perform and can be used irrespective of the pathology that is contained in the
specimen. It allows inspection of the entire specimen surface, ie. of all circumferential
resection margins in every single specimen slice. The resulting axial specimen slices are
easy to sample from, either as standard or whole-mount tissue blocks.
Figure 2. Serial slicing of a pancreatoduodenectomy specimen along the axial plane.
To facilitate axial slicing, it is helpful to remove the the gall bladder and the 'tail' of duodenum
below the level of the pancreatic head. It is therefore recommended to take the following
tissue samples prior to axial slicing: the proximal (gastric or duodenal) and distal (duodenal)
transection margins, the pancreatic transection margin, the transection margin of the
common bile duct, the gall bladder and cystic duct.
Subsequently, the specimen can be sliced in the axial plane (ie. perpendicular to the
longitudinal duodenal axis) through the entire pancreatic head (figure 2). The specimen
slices should be no more than 3 mm thick, as some of the native anatomical structures are of
that order of magnitude (eg. normal main pancreatic duct: 2-3 mm diameter). For most cases
this will result in at least 12 slices. The specimen slices should be laid out in sequential order,
the most cranial at the top, with the caudal cut surface showing upward ('looking from below'
as on CT imaging (figure 3). Photographs should be taken at this stage, including an
overview picture of all slices and a viewer-filling close-up image of at least those slices that
contain the cancer and any other lesion(s).
Sida 6 av 20
Figure 3. Display of axial slices from a pancreatoduodenectomy specimen: 16 serial
specimen slices are lined up from the cranial end (left upper corner) to the caudal limit of the
pancreatic head (right lower corner). The entire course of the common bile duct (CBD) can
be followed from the top slice to the ampulla (AMP). The normally-sized main pancreatic duct
(MPD) is also visualised. Note the presence of a small cancer (CAN) at and inferior to the
ampulla, which is well clear of the anterior surface (ANT; inked red), and the SMV (green),
SMA (yellow) and posterior (POS; blue) resection margins. An area of fat necrosis is present
in the anterior peripancreatic tissue at and caudal to the level of the ampulla.
Distal pancreatectomy specimens should be dissected by serial sectioning in 3 mm thick
slices along a sagittal plane, ie. perpendicular to the longitudinal axis of the pancreatic body.
For total pancreatectomy specimens a combined approach of axial slicing of the pancreatic
head and sagittal slicing of the pancreatic body and tail is recommended.
The pancreatic head is the part of the pancreas to the right of the left border of the SMV. The
uncinate process is considered part of the pancreatic head. The pancreatic body lies
between the left border of the aorta and the splenic hilum.
4. Inspection of the dissected specimen
The tumour is described regarding its appearance (eg. colour, texture, demarcation) and
location. The latter is of utmost importance for clinicopathological correlation and
identification of the cancer origin (ie. pancreas, ampulla or common bile duct) (3). The
location within the pancreatic head (eg. medial-dorsal half, lateral-anterior aspect), and the
spatial relationship to the key anatomical structures (ampulla, duodenal wall and intra/extrapancreatic bile duct, peripancreatic soft tissue, SMV if resected, etc) are recorded. The
craniocaudal extension of the tumour is indicated by recording the slices that are deemed to
contain tumour (eg. slice 3 to 8). The craniocaudal length of the tumour can be derived from
simple calculation (craniocaudal length of the pancreatic head divided by the total number of
specimen slices, multiplied by the number of tumour-involved slices). The two tumour
dimensions in the axial plane are measured in the specimen slice where the tumour is at its
largest extension. The minimum distance of the tumour to the nearest specimen margins and
surfaces is assessed at this point, however, this requires microscopic confirmation and exact
measurement. Any further abnormalities, identified in the pancreas or other structures
included in the specimen are described.
Sida 7 av 20
5. Tissue sampling
It is recommended to take at least one whole mount sample from the specimen slice in which
the tumour is at its largest and which demonstrates best the relationship to structures that
are essential for correct T- and R-staging. The staging criteria for pancreatic, ampullary,
distal bile duct and duodenal cancer differ and need separate consideration (4). As cancer in
the pancreatic head is usually poorly circumscribed (5), and therefore the relationship of the
invasive tumour front to other structures and the resection margins is often difficult to assess,
extensive sampling is recommended. The number of tumour samples has a direct impact on
correct assessment of the margin status (6). To allow verification and correction of the
craniocaudal dimension of the tumour, samples should also be taken from the specimen
slices cranial and caudal to those containing the macroscopically apparent top and bottom
end of the tumour (7). Further samples may be needed if additional tissues or structures (eg.
venous segment) are included in the specimen, to examine the relationship of these to the
tumour. Tissue samples should also be taken from background pancreatic parenchyma,
ampulla and bile duct.
6. Lymph node sampling and allocation
The lymph nodes are not dissected out from the specimen, but instead they are left intact
and sampled together with the surrounding tissues. The colour of the ink on the specimen
surface overlying the lymph nodes will allow allocation to the different lymph node stations
defined by the UICC or the Japan Pancreatic Society (JPS), as illustrated in table 1 and
figure 4. Perigastric lymph nodes can be dissected from the perigastric adipose tissue along
the greater and lesser curve and embedded separately. On occasion, one or two lymph
nodes may be found in the sparse adipose tissue adherent to the distal duodenum included
in a Whipple's specimen.
It has to be noted that according to TNM 7. edition, the exact lymph node station is of no
significance for staging, in as far as tumour involvement of any of the regional lymph node
stations is to be reported as N1 (4). Which lymph nodes are regarded as regional for the
different tumour localisations is summarised in table 2. As part of a surgical resection
procedure, additional lymph node stations may be received separately, eg. lymph nodes from
station 8, the coeliac trunc or aortocaval. The latter lymph nodes are non-regional, and
therefore their involvement represents M1, not N1. In contrast, lymph nodes from the
hepatoduodenal ligament, the coeliac trunc and station 8 represent regional lymph nodes for
cancer of the pancreatic head, while lymph nodes from the splenic hilum are regional
drainage stations for cancer of the pancreatic body and tail (4).
A Whipple's resection specimen yields on average a minimum of 15 lymph nodes, and this
has been accepted as a quality benchmark (8), ahe number of evaluated lymph nodes
influences survival (9-10).
Sida 8 av 20
Table 1 (4,11): Classification of lymph node stations according to Japan Pancreas
Society (JPS) and UICC
JPS lymph
node stations
6
Equivalent UICC lymph
node stations
Infrapyloric
8
Common hepatic artery
9
Coeliac
10
11
Splenic hilum
Superior/along splenic artery
12
Hepatoduodenal ligament
(portal/bile duct)
13
14
16
Posterior pancreatoduodenal
Superior mesenteric vessel
Para-aortic
17
Anterior pancreatoduodenal
18
Inferior
Position in specimen slices from PDE
or DPE
Superior’: in slices cranial to top end of
pancreatic head
NA (not included in specimen; received
separately)
NA (not included in specimen; received
separately)
Splenic hilum
Along superior border of pancreatic
body/tail
Along extrapancreatic common bile duct
stump (12b2 = along bile duct); or not
included in specimen and received
separately (other stations 12)
Along posterior margin
Along SMV (14A) and SMA margins (14V)
NA (not included in specimen; received
separately)
Along anterior surface
‘Inferior’: in slices caudal to bottom end of
pancreatic head
Abbreviations: DPE: distal pancreatectomy; JPS: Japan Pancreas Society; NA: not
applicable; PDE: pancreatoduodenectomy; SMA: superior mesenteric artery; SMV: superior
mesenteric vein; UICC: Union International Contre le Cancer.
Table 2 (4): Regional lymph node stations for pancreatic, ampullary, distal bile duct
and duodenal cancer
Lymph node station
Pancreas
Ampulla
Superior (11)
Inferior (18)
Anterior pancreatoduodenal (17)
Posterior pancreatoduodenal (13)
Hepatoduodenal ligament
(portal/bile duct) (12)
Superior mesenteric vessel (14)
Splenic hilum (10)
x
x
x
x
x
x
x
x
x
x
x
x (only body &
tail)
x (only head)
x
Coeliac (9)
x
Common bile
duct
x
Duodenum
x
x
x
x
x
x
x
x
x
Sida 9 av 20
Figure 4. Localisation of the various lymph node stations according to the
Japan Pancreas Society (JPS) (11,12).
IV. Processing of pancreatic biopsies
The number and length of the trucut biopsies, and possible tissue fragmentation are
recorded. The biopsy sample is embedded in paraffin, cut and stained with HE. Multiple
spare sections are made and preserved for special stains that may be required.
V. Information to be included in the pathology report
1. Ductal adenocarcinoma and variants
The histological tumour type is stated according to the WHO classification (13). Ductal
adenocarcinoma, which represents approximately 89% of all pancreatic tumours, is graded
as well, moderately and poorly differentiated, according to criteria outlined in table 3. The
tumour is graded according to the least differentiated area, regardless of prevalence
(13,14,15).
Sida 10 av 20
Table 3 (13, 14): Histopathological grading of ductal adenocarcinoma of the pancreas *
Tumour
Glandular
differentiation
Mucin
production
Grade
Mitoses
Grade 1
Well-differentiated
Intensive
(per 10
HPF)
5
Grade 2
Moderately
differentiated ductlike structures and
tubular glands
Poorly
differentiated
glands, abortive
mucoepidermoid
and pleomorphic
structures
Irregular
6-10
Abortive
>10
Grade 3
Nuclear features
Little polymorphism,
polar arrangement
Moderate
polymorphism
Marked pleomorphism
and increased size
*Grade is assigned on the basis of the feature of highest grade (14)
Variants of ductal adenocarcinoma include adenosquamous carcinoma, colloid
adenocarcinoma, medullary carcinoma, undifferentiated carcinoma, and undifferentiated
carcinoma with osteoclast-like giant cells (13).
Tumour staging is reported according the AJCC/UICC TNM staging system (7. edition; see
Appendix 1) (4). Note that for pancreatic cancer, infiltration of the intrapancreatic bile duct
represents stage pT3.
The presence of perineural, lymphatic and vascular tumour propagation is recorded. Invasive
carcinoma present at less than 1 mm from a resection margin is reported as R1.
(1,2,6,16,17). As the anterior pancreatic surface represents a true anatomical surface rather
than a resection margin, this surface has to be breached by invasive tumour (0 mm
clearance) for this surface to be considered involved (2,8). It is recommended not to use the
terminology ‘radical/non-radical’ or ‘curative/non-curative’. The former refers to a different,
more extensive surgical procedure, while regarding the latter, it must be borne in mind that
pancreatic cancer resection, even if R0, hardly ever cures the patient.
Reporting on resection specimens for pancreatic ductal adenocarcinoma following neoadjuvant treatment should include an assessment of the degree of tumour regression. While
multiple systems for this have been proposed (overview in 18), the use of a 4-tiered grading
system for the extent of residual carcinoma following neo-adjuvant treatment is
recommended (table 4), because it is simple and easy to apply (19). Following neo-adjuvant
treatment, the grade of differentiation of residual cancer is not evaluated, as this is affected
by treatment-induced changes. There is currently no adequate, evidence-based criterion for
reporting of the margin status. It is therefore best to avoid categorically diagnosing R0 or R1,
but to merely state the minimum clearance instead.
Sida 11 av 20
Table 4 (adapted from 19):
Grading of regression of pancreatic cancer following neo-adjuvant treatment
Grade
0
1
2
3
Proportion of residual viable tumour tissue
No residual viable tumour (histologic complete response)
Marked response: minimal residual cancer with single cells or small groups
of cancer cells
Moderate response: residual cancer outgrown by fibrosis
Poor / no response: extensive residual cancer.
Differential diagnosis:
- chronic (obstructive) pancreatitis: Distinction between invasive carcinoma and reactive
ducts and acinar structures can occasionally be problematic, especially in frozen sections,
pancreatic biopsies or in the pancreatic transection margin. To prevent the latter diagnostic
difficulty, sampling of the tissue slice immediately adjacent to the true resection margin may
prove helpful, as the latter is often of poor quality, affected by crush and/or diathermy
artefact.
Findings that suggest invasive adenocarcinoma are (20):
- irregular distribution of ducts and glands, ie. without recognisable lobular
arrangement
- glands in immediate vicinity of muscular vessels
- perineural or vascular tumour propagation
- glands with an incomplete lumen
- single cells
- 4:1 rule: anisonucleosis with at least a 4-fold difference in nuclear size between cells
lining the same glandular structure
- large irregular nucleoli
- intraluminal necrotic debris
- mitotic figures, especially if atypical.
The vast majority of ductal adenocarcinomas express cytokeratins 7, 8, 18 and 19 (table 5).
Other markers that can be detected in adenocarcinoma are CEA (mono- and polyclonal),
CA19-9 and CA125. Immunostaining for SMAD4 is negative in approximately 55% of ductal
adenocarcinomas and positive in reactive ducts. Immuostaining of P53 is present of 50-75%
of ductal adenocarcinomas, whereas in 90% of these cancers, immunolabelling for P16 is
absent. It has to be kept in mind, however, that these markers may not always be reliable for
the distinction between reactive changes and cancer when applied to a single gland or a
small cluster of glands (eg. in biopsy material).
Table 5:
Immunohistochemical profile of ductal adenocarcinoma of the pancreas (19)
Antibody
CK7
CK8, CK18
CK19
CK20
MUC1
MUC2
MUC5AC
MUC6
Percentage positive in ductal adenocarcinoma of the pancreas
100
100
100
28
87
9
70
24
Sida 12 av 20
In most cases, chronic inflammatory changes, atrophy and fibrosis observed in a pancreatic
cancer resection specimen will represent obstructive pancreatitis rather than pre-existing true
chronic pancreatitis. To avoid confusion and erroneous interpretation, changes of obstructive
pancreatitis should not be merely reported as ‘chronic pancreatitis’, as this could be
misinterpreted by clinical colleagues as pre-existing chronic pancreatitis, ie. a precursor
condition of pancreatic cancer.
- ampullary, bile duct or duodenal adenocarcinoma: this important distinction is almost
exclusively based on macroscopic findings, ie. the localization of the epicenter of the tumour
(3). It should be borne in mind that ductal adenocarcinoma of the pancreas is believed to
arise from peripheral ramifications of the pancreatic duct system, not from the main
pancreatic duct (unless in the context of IPMN, see below). As the histology of the main
pancreatic duct and bile duct is not dissimilar, care has to be taken not to report a bile duct
cancer growing in and around the intrapancreatic bile duct as a ductal adenocarcinoma of
the pancreas.
The presence of a precursor lesion can be helpful. This is most frequently found in the form
of an adenoma in association with ampullary cancer (up to over 80%). In contrast, precursor
neoplasia in association with distal bile duct cancer is much less common (10-33%), and
usually presents as flat dysplasia rather than a polypoid lesion. The diagnostic value of
pancreatic intraepithelial neoplasia as evidence of pancreatic origin of adenocarcinoma is
limited, because PanIN-1 or -2 is a common finding in the general population, especially over
the age of 50 years, and can be fortuitously coexistent with non-pancreatic cancer.
Intestinal or pancreatobiliary differentiation does not only occur in ampullary, but also in distal
bile duct cancer (13). A small proportion of pancreatic cancers have also been described to
be of intestinal differentiation (22). Hence, this feature does not allow a definitive distinction
between the three cancer groups. Intestinal differentiated adenocarcinoma tends to be CK20,
CDX2 and MUC2 positive, whereas pancreatobiliary differentiated tumours are usually CK7
and MUC1 positive, but negative for CDX2.
2. Pancreatic intraepithelial neoplasia (PanIN)
This lesion is defined as a non-invasive epithelial proliferation in small pancreatic ducts,
which can be premalignant and develop into invasive ductal adenocarcinoma. Earlier
nomenclature, including ductal hyperplasia, hypertrophy, metaplasia or dysplasia should no
longer be used. PanIN is divided into (13,23):
PanIN-1A/B: Minimal cytological and architectural atypia. High columnar mucinous
epithelium with round to ovoid, basally placed nuclei. No mitotic figures. PanIN-1A lesions
are flat, PanIN-1B papillary in architecture.
PanIN-2: Moderate cytological and architectural atypia. The epithelial lining is more cellular,
and shows disturbed cellular polarity, pseudostratification, and enlarged and hyperchromatic
nuclei. Occasional typical and non-luminal mitotic figures may be present. Usually papillary in
architecture.
PanIN-3: Marked cytological and architectural atypia. Large hyperchromatic nuclei,
pronounced loss of cellular polarity, dystrophic goblet cells, prominent nucleoli, atypical
and/or luminal mitotic figures. Commonly (micro-)papillary architecture with possible
cribriform structures and intraluminal necrotic debris.
The differential diagnosis mainly includes reactive duct-epithelial changes, invasive
adenocarcinoma (especially in biopsy material), and duct cancerisation. PanIN is a
Sida 13 av 20
microscopic lesion, which usually does not exhibit true papillae and is of a gastric, not an
intestinal type (MUC1, MUC5AC and MUC6 positive, MUC2 negative). The latter features
are helpful in distinguishing PanIN from IPMN (see below) (24).
The presence of PanIN-3 at the transection margin should be reported, although the
implications of this finding on patient management are not clear at present. According to a
recent study, the presence of PanIN-3 at the transection margin has no prognostic
implications, if the pancreatic resection was performed for ductal adenocarcinoma (25).
As PanIN-1 and -2 are common in the general population (26), in particularly in the age
group over 50, the presence of these lesions cannot be used as evidence for a pancreatic
origin of a pancreatic head carcinoma.
3. Intraductal papillary mucinous neoplasia (IPMN)
This lesion is – as its name implies – characterised by an intraductal neoplastic proliferation
of mucin-producing epithelium, which grows in papillary formations. The latter two features,
however, can vary, and areas of minimal mucin production or papillary tufting can be
encountered. The lesion can involve the main pancreatic duct, side branch ducts, or both. It
can present as a localized lesion, but is often multifocal and may involve large parts of, or
occasionally the entire, pancreatic duct system. IPMN lesions require extensive sampling
and examination, sometimes of the entire lesion, to exclude transition into invasive
adenocarcinoma. Any solid or mucinous areas should be prioritised for sampling. Complete
embedding may be required in some cases, in particular in those showing extensive severe
dysplasia (27).
The distinction between IPMNs of a main duct type, a branch duct type, or a combined type
is based on combined macro- and microscopic examination. Current evidence shows that
branch duct type IPMN has a better prognosis than the main duct type.
Microscopically, IPMN are classified according to the type of neoplastic epithelium: intestinal,
gastric (foveolar), pancreatobiliary and the rare oncocytic type (13) (table 6). Different
epithelial types can be found within the same lesion. The gastric type is commonly seen in
branch duct type IPMN.
Table 6 (modified from 13): Differential morphology and immunolabelling of IPMN
Type
Intestinal
Gastric
Pancreatobiliary
Oncocytic
Histology
Villous architecture, cigar-shaped
nuclei, apical mucin, goblet cells.
Weakly eosinophilic epithelium, basal
nuclei, apical mucin. Often only mild
dysplasia.
Complex arborising papillae,
occasional cribriform architecture,
round nuclei with prominent nucleoli.
Usually severe dysplasia.
Complex papillae with transition into
cribriform or solid structures,
intraepithelial lumina, oncocytic
epithelium. Usually severe dysplasia.
Immunohistochemistry
+ MUC2 and MUC5AC, CDX2
- MUC1, MUC6
+ MUC5AC
- MUC1, MUC2, MUC5AC,
MUC6, CDX2
+ MUC1, MUC5AC, MUC6
- MUC2, CDX2
+ MUC1 and MUC6
- MUC2, MUC5AC, CDX2
The grade of dysplasia of IPMNs is determined by the degree of cytological and architectural
atypia and reported as mild, moderate or severe (carcinoma in situ). The previous terms ‘IPM
adenoma’ and ’borderline IPMN’ should no longer be used.
The grade of dysplasia of IPMN is of key importance for intra-operative frozen section
examination of the pancreatic transection margin. The presence of severely dysplastic IPMN
Sida 14 av 20
may cause consideration of extended surgical resection. Occasionally, epithelial lining of the
ducts represented in the frozen section is largely absent, in which case all options to
visualise any residual epithelium should be exhausted (deeper section levels, turning the
tissue block over 180 degrees). In case these fail, the possibility of examining a new tissue
sample should be discussed with the surgeon (27,28).
IPMN is a macroscopically detectable lesion, which microscopically includes true, welldeveloped papillary structures with a fibrovascular core. The presence of copious luminal
mucin and positive immunohistochemical labelling for MUC2 favour IPMN over PanIN (23).
Occasional lesions that are problematic to categorise (eg. those of gastric type with a degree
of papillary folding involving small branch ducts) are best merely described by their size and
degree of dysplasia. Further included in the differential diagnosis of IPMN is mucinous cystic
neoplasia (see below).
Malignant transformation of IPMN can result in conventional adenocarcinoma or mucinous
(‘colloid’) adenocarcinoma. The latter has a better outcome and is associated with intestinal
type IPMN, whereas conventional ductal adenocarcinoma results from malignant
transformation of the pancreatobiliary or gastric type.
Recently, another tumour entity that is characterised by intraductal growth, has been
introduced: intraductal tubulopapillary neoplasm (ITPN). Unlike IPMN, this tumour does not
usually produce copious mucin and it tends to present as several fairly well-circumscribed
solid nodular masses within dilated ducts. Histologically, these show a predominant tubular
or cribriform growth pattern with few if any papillary structures, and are usually high-grade
dysplastic. Associated invasive carcinoma is found in ca. 40% of cases (13).
4. Mucinous cystic neoplasm (MCN)
More than 95 % of patients with MCN are female, and often of a younger age than patients
suffering from IPMN or ductal adenocarcinoma. MCN is much more common in the
pancreatic body and tail than in the head region. MCN is a solitary tumour without
communication with the pancreatic duct system. A key diagnostic charecteristic of MCN is
the presence of ovarian-type stroma, which on immunostaining is positive for oestrogen
receptor protein (25% of cases) or progesteron receptor (50-75%). MCNs are usually wellcircumscribed and surrounded by a layer of fibrous tissue of varying width. Dysplasia of the
neoplastic mucinous epithelium is graded as mild, moderate and severe (carcinoma in situ).
The previous terms ‘MCN adenoma’ and ’borderline MCN’ should no longer be used.
Over time, MCN can transform into invasive adenocarcinoma, and therefore, sampling
should be performed as outlined for IPMN (28,29).
5. Serous cystadenoma (SCA)
This cystic tumour is usually well-circumscribed and can reach a considerable size (mean
diameter: 6 cm). The microcystic SCA, the most common form, is composed of numerous
small watery cysts with paper-thin walls, which are smaller in the centre and often arranged
around a central stellate scar that can be calcified. Microscopically, the cysts are lined by a
single layer of columnar cells with a central uniform round nucleus and clear, glycogen-rich
cytoplasm (PAS positive, PAS-diastase negative) that stains for epithelial markers (EMA and
CK7,8,18,19), but is negative for neuroendocrine markers and CEA. Cytological atypia or
mitotic figures are not seen, however, occasional focal mild papillary tufting of cyst-lining
epithelium can occur. Identical histology is seen in the less common macrocystic SCA, which
is composed of a smaller number of larger cysts. The rare solid variant is composed of the
same neoplastic epithelium, which lines tightly packed microtubular structures. SCA are
benign but can be associated with von Hippel-Lindau syndrome, which overall is associated
with a reduced life expectancy (28,29).
Sida 15 av 20
6. Solid-pseudopapillary tumour (SPT)
This tumour affects mainly females in their 20s and 30s. SPTs are usually large, wellcircumscribed tumours, composed of soft solid tissue, which frequently exhibits pseudocystic
change and haemorrhage. Microscopically, they consist of solid sheets of medium-sized
monomorphic tumour cells with a uniform ovoid nucleus and occasional nuclear grooves.
Degenerative changes are prominent and lead to the formation of pseudopapillae. Endocrine
tumours are the main differential, which can be resolved immunohistochemically, as
vimentin, alpha-1-antitrypsin, progesterone receptor, beta-catenin (nuclear), NSE, and CD56
are are expressed, while staining for synaptophysin and chromogranin A is positive only in a
proportion of tumour cells (20). Even if the tumour recurs or metastasizes (in 10-15% of
patients), survival is usually long-term. There are no proven morphological predictors of
outcome.
7. Endocrine tumours and mixed adenoneuroendocrine carcinoma (MANEC)
Please, see the KVAST-document ’Endokrina tumörer i mag-tarmkanal och pankreas’.
8. Acinar cell carcinoma
This uncommon aggressive tumour affects mainly adults, but has also been reported in
children. A minority of patients have a paraneoplastic syndrome characterised by lipase
hypersecretion. It is a solid, well-circumscribed and usually large tumour, which histologically
is chararcterised by a lobulated architecture. Except for the fibrous bands between the
tumour lobules, stroma is usually scanty in this tumour. The composing cells have a
moderate amount of finely granular cytoplasm and remarkably uniform nuclei with a single
prominent central nucleolus. In many, especially not so well differentiated cases,
immunohistochemistry is required to reach a conclusive diagnosis (trypsin, chymotrypsin,
lipase positive in 66-90%). An overview of the immunohistochemical profile of common
pancreatic neoplasms is given in Table 7.
Table 7 (modified from 13): Immunohistochemical profile of common pancreatic
neoplasms
Marker
Ductal
neoplasms*
PEN
Acinar cell
carcinoma
CK8/18
CK19
Vimentin
Trypsin/chymotrypsin
Chromogranin
Synaptophysin
CD10
β-catenin (nuclear)
++
++
F
F
+
-
++
+
++
++
-
++
++
F
F
+
Solidpseudopapillary
tumour
F
+
+
++
++
Abbreviations: ++: usually positive; +: may be positive; F: may be focally positive; -: usually
negative; PEN: pancreatic endocrine neoplasm
*: ductal neoplasms include invasive ductal adenocarcinoma, mucinous cystic neoplasm,
intraductal papillary mucinous neoplasm, and serous cystic
neoplasms.
Sida 16 av 20
9. Chronic pancreatitis
Chronic pancreatitis is characterised by a combination of changes: acinar atrophy, fibrosis,
and clustering of 'naked' islets that may be enlarged to the point of mimicing endocrine
neoplasia. Chronic inflammatory cell infiltration is usually mild and patchy, and not
uncommonly centred on peripheral nerves, which are often prominently enlarged and
numerous. Morphology usually does not allow identification of the aetiology, ie. alcoholrelated, tropical, hereditary, idiopathic. Forms of pancreatitis with distinctive morphological
features are (30):
Autoimmune pancreatitis: fibro-inflammatory disease of presumed autoimmune aetiology that
can be part of a multi-organ disorder. The pancreas can be affected diffusely or focally. The
inflammatory proces centres on main and interlobular pancreatic ducts, which are deformed,
and whose epithelium may be folded, detached or destroyed. There are two histological
types. In type, 1 the inflammatory infiltrate contains numerous plasma cells (many of which
are IgG4+) and scattered eosinophils, and phlebitis is usually present. In type 2, numerous
neutrophils are present, which cause epithelial/ductal destruction and microabscesses.
Phlebitis is usually absent and IgG4+ plasma cells are rare. Common to both types is a
patchy distribution of changes within the affected pancreatic area.
Groove pancreatitis (syn. paraduodenal pancreatitis, cystic dystrophy of the duodenum,
paraduodenal wall cysts): inflammatory changes are limited to the duodenal wall facing the
pancreas and the adjacent pancreatic parenchyma ('pancreatoduodenal groove'), around
and superior to the ampulla of Vater. The duodenal wall is irregularly thickened and
indurated, and it often contains one or multiple cystic cavities. Inflammation extends into the
periduodenal and adjacent pancreatic tissue. Histologically, ectopic pancreatic tissue is
present within the duodenal wall, and (cystically) dilated ducts are present, some of which
have ruptured and caused acute and chronic inflammation in the surrounding duodenal and
pancreatic tissues. Alcohol is a precipitating factor.
Pseudocysts are a common complication of both acute and chronic pancreatitis. They are
devoid of epithelial lining and their wall consists of inflammatory tissue. A connection
between the lumen and pancreatic duct system is often not identifiable.
VI. Recommendations for reporting
The use of a report proforma may be considered and found helpful to ensure comprehensive
and uniform reporting (link to the Karolinska Universitetslaboratoriet proforma for the
reporting of tumours of the pancreas and periampullary region).
VII. SNOMED-codes
T 59000 pancreas
T 59100 head of pancreas
T 59200 body of pancreas
T 59300 tail of pancreas
T 99000 endocrine pancreas
T 58700 ampulla of Vater
T 58000 extrahepatic bile ducts
T 58500 common bile duct
T 64000 duodenum
M 81403 adenocarcinoma
M 85003 ductal adenocarcinoma
M 85603 adenosquamous carcinoma
Sida 17 av 20
M 84803 colloid (mucinous non-cystic) carcinoma
M 85103 medullary carcinoma
M 80203 undifferentiated carcinoma
M 43000 chronic pancreatitis
M 69726 pancreatic intraepithelial neoplasia (PanIN-1A/B)
M 69727 pancreatic intraepithelial neoplasia (PanIN-2)
M 69728 pancreatic intraepithelial neoplasia (PanIN-3)
M 84530 intraductal papillary mucinous neoplasia (IPMN), mild dysplasia
M 84531 intraductal papillary mucinous neoplasia (IPMN), moderate dysplasia
M 84532 intraductal papillary mucinous neoplasia (IPMN), severe dysplasia (carcinoma in
situ)
M 84532 + M 85003 intraductal papillary mucinous neoplasia (IPMN), severe dysplasia with
transition in invasive carcinoma
M 84700 mucinous cystic neoplasia, mild dysplasia
M 84721 mucinous cystic neoplasia, moderate dysplasia
M 84703 mucinous cystic neoplasia, severe dysplasia (carcinoma in situ)
M 84410 microcystic serous adenoma
M 85503 acinar cell carcinoma
M 89713 pancreatoblastoma
M 84523 solid-pseudopapillary tumour
M 85032 intraductal tubulopapillary neoplasm (ITPN)
M 33400 pseudocyst
M40000 + D47000 autoimmune pancreatitis
VIII. References
1. Jamieson NB, Glen P, Oien KA, Going JJ, Foulis AK, Dickson EJ, Imrie CW, Mckay CJ,
Carter R. Positive mobilization margins alone do not influence survival following
pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. Ann Surg 2010;
251:1003-1010.
2. Verbeke CS. Resection margins and R1 rates in pancreatic cancer- are we there yet?
Histopathology 2008; 52:787-796.
3. Verbeke CS, Gladhaug IP. Resection margin involvement and tumour origin in pancreatic
head cancer. BJS (Epub ahead of print, Apr 20; 2011).
4. Sobin LH, Gospodarowicz MK, Wittekind C (eds) (2009). UICC: TNM classification of
malignant tumours 7th edn. Wiley-Blackwell, Oxford.
5. Verbeke CS, Knapp J, Gladhaug IP. Tumour growth is more dispersed in pancreatic head
cancers than in rectal cancer: implications for resection margin assessment. Histopathology
2011;59:1111-1121.
6. Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the
R1 resection in pancreatic cancer. BJS 2006; 93:1232-1237.
7. Verbeke C, Sheridan M, Scarsbrook A, Albazaz R, Smith A, Menon K. Guthrie A. How
accurate is size assessment of pancreatic head cancers by radiology and pathology?
Pancreatology 2010; 140:300.
8. Campbell F, Foulis AK, Verbeke CS. Dataset for the histopathological reporting of carcinomas
of the pancreas, ampulla of Vater and common bile duct. Royal College of Pathologists,
www.rcpath.org (2010).
9. Sierzega M, Popiela T, Kulig J, Nowak K. The ratio of metastatic/resected lymph nodes is
an independent prognostic factor in patients with node-positive pancreatic head cancer.
Pancreas 2006; 33:240-245.
10. Tomlinson J, Jain S, Bentrem J et al. Accuracy of Staging Node-Negative Pancreas
Cancer. Arch Surg 2007; 142:767-774.
11. Japanese Pancreas Society. Classification of pancreatic carcinoma. 2nd English ed. Tokyo:
Kanehara & Co, Ltd (2003).
Sida 18 av 20
12. Roche CJ, Hughes ML, Garvey CJ, Campbell F, White DA, Jones L, Neoptolemos JP.
CT and pathological assessment of prospective nodal staging in patients with ductal adenocarcinoma
of the head of pancreas. AJR 2003;180:475-480.
13. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system,
4th edition. International Agency for Research on Cancer, Lyon (2010).
14. Klöppel G, Lingenthal G, von Bulow M, Kern HF. Histological and fine structural features of pancreatic
ductal adenocarcinomas in relation to growth and prognosis: studies in xenografted tumors and
clinicopathological correlation in a series of 65 cases. Histopathology 1985;9:841-856.
15. Lüttges J, Schemm S, Vogel I, Hedderich J, Kremer B, Klöppel G. The grade of ductal pancreatic
adenocarcinoma is an independent prognostic factor and is superior to the immunohistochemical
assessment of proliferation. J Pathol 2000;191:154-161.
16. Esposito I, Kleeff J, Bergmann F et al. Most pancreatic resections are R1 resections. Ann
Surg Oncol 2008;15:1651-1660.
17. Campbell F, Smith RA, Whelan P et al. Classification of R1 resections for pancreatic
cancer: the prognostic relevance of tumour involvement within 1 mm of a resection margin.
Histopathology 2009;55:277-283.
18. Chatterjee D, Katz MH, Rashid A et al. Histologic grading the extent of residual
carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma. A
predictor for patient outcome. Cancer (Epub ahead of print; Oct 25, 2011).
19. Washington K, Berlin J, Branton P et al. Protocol for the examination of specimens from
patients with carcinoma of the exocrine pancreas. Northfiled, IL: College of American
Pathologists; 2010.
20. Hruban RH, Bishop Pitman M, Klimsta DS. Tumors of the Pancreas. AFIP Atlas of Tumor Pathology,
Series 4. ARP Press (2007).
21. Lee MJ, Lee HS, Kim WH, Choi Y, Yang M. Expression of mucins and cytokeratins in
primary carcinomas of the digestive system. Mod Pathol 2003;16:403-1410.
22. Albores-Saavedra, Simpson K, Dancer Y-J, Hruban R. Intestinal type adenocarcinoma: a
previously unrecognized histologic variant of ductal carcinoma of the pancreas. Ann Diagn
Pathol 2007;11:3-9.
23. Hruban RH, Adsay NV, Albores-Saveedra J et al. Pancreatic intraepithelial neoplasia: a
new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol
2001;25:579-586.
23. Hruban RH, Takaori K, Klimstra DS et al. An illustrated consensus on the classification of
pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg
Pathol 2004;28:977-987.
24. Matthaei H, Hong S-M, Mayo SC et al. Presence of pancreatic intraepithelial neoplasia in
the pancreatic transection margin does not influence outcome in patients with R0 resected
pancreatic cancer. Ann Surg Oncol 2011;18:3493-3499.
25. Sipos B, Frank S, Gress T, Hahn S, Klöppel G. Pancreatic intraepithelial neoplasia
revisited and updated. Pancreatology 2009;9:45-54.
26. Katabi N, Klimstra DS. Intraductal papillary mucinous neoplasms of the pancreas: clinical
and pathological features and diagnostic approach. J Clin Pathol 2008;61:1303-1313.
27. Sauvanet A, Couvelard A, Belghiti J. Role of frozen section assessment for intraductal
papilalry and mucinous tumor of the pancreas. WJGS 2010;2:352-358.
Cioc AM, Ellison EC, Proca DM et al. Frozen section diagnosis of pancreatic lesions. Arch
Pathol Lab Med 2002;126:1169-1173.
28. Campbell F, Azadeh B. Cystic neoplasms of the exocrine pancreas. Histopathology
2008;52:539-551.
29. Basturk O, Coban I, Adsay NV. Pancreatic cysts. Pathologic classification, differential
diagnosis, and clinical implications. Arch Pathol Lab Med 2009;133:423-438.
30. Klöppel G, Adsay NV. Chronic pancreatitis and the differential diagnosis versus
pancreatic cancer. Arch Pathol Lab Med 2009;133:382-387.
Sida 19 av 20
IX. List of abbreviations
KVAST: Kvalitets- och standardiseringskommittén
IPMN: intraductal papillary mucinous neoplasm
MCN: mucinous cystic neoplasm
SMA: superior mesenteric artery
SMV: superior mesenteric vein
X. Membership of the KVAST study group
Name
Telephone
E-mail
Mikael Björnstedt
+46 (0)8 5858 3809
Mikael.Bjornstedt@ki.se
Lennart Franzén
+46 (0)7 0839 1500
Lennart.Franzen@aleris.se
Hans Glaumann
+46 (0)8 5858 0000
Glaumann@telia.com
Hans Nordlinder
+46 (0)3 1342 2819
Hans.Nordlinder@vgregion.se
Richard Palmqvist
+46 (0)9 0785 0000
Richard.Palmqvist@medbio.umu.se
Pehr Rissler
+46 (0)4 6173436
Pehr.Rissler@skane.se
+46 (0)7 0977 5561
Åke Öst
+46 (0)7 33929334
Ake.ost@comhem.se
Caroline Verbeke
+46 (0)7 6907 6732
Caroline.Verbeke@ki.se
Sida 20 av 20
APPENDIX 1
pTNM classification (according to 7th edition) (p for pathology)
TX Primary tumour cannot be assessed
T0 No evidence of primary tumour
Tis Carcinoma in situ (for pancreas, this also includes PanIN-3)
NX Regional lymph nodes cannot be assessed
N0 No regional lymph node metastasis
N1 Regional lymph node metastasis
MX Distant metastasis cannot be assessed
M0 No distant metastasis
M1 Distant metastasis (metastasis to non-regional lymph nodes is recorded as M1).
Pancreas
T1 Tumour limited to pancreas, 2 cm or less in greatest dimension
T2 Tumour limited to pancreas, more than 2 cm in greatest dimension
T3 Tumour extends beyond pancreas, but without involvement of coeliac axis or superior
mesenteric artery
T4 Tumour involves coeliac axis or superior mesenteric artery
Ampulla of Vater
T1 Tumour limited to ampulla of Vater or sphincter of Oddi
T2 Tumour invades duodenal wall
T3 Tumour invades pancreas
T4 Tumour invades peripancreatic soft tissues, or other adjacent organs or structures
Extrahepatic bile ducts-distal
T1 Tumour confined to bile duct
T2 Tumour invades beyond wall of bile duct
T3 Tumour invades the gall bladder, liver, pancreas, duodenum, or other adjacent organs
T4 Tumour involves coeliac axis or superior mesenteric artery
Small bowel (including duodenum)
T1a Tumor invades lamina propria or muscularis mucosae
T1b Tumor invadas submucosa
T2 Tumor invades muscularis propria
T3 Tumor invades subserosa or non-peritonealized perimuscular tissue (mesentery or
retroperitoneum)
with extension 2 cm or less
T4 Tumour perforates visceral peritoneum or directly invades other organs or structures
(includes other
loops of small intestine, mesentery, or retroperitoneum more than 2 cm and abdominal wall by
way of
for duodenum only, invasion of pancreas)
N1 Metastasis in 1-3 regional lymph nodes
N2 Metastasis in 4 or more regional lymph nodes