Quality Quotes
advertisement

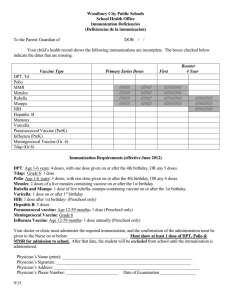
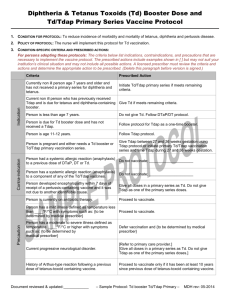
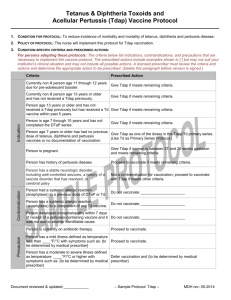
Quality Quotes Immunization Updates The new 2006 Immunization Schedule for Children and Adolescents was released on January 9, 2006, and can be accessed at either of the following websites: www.cdc.gov/nip or www. aap.org. The 2006 schedule refl ects several changes from the 2005 schedule including the following: Hepatitis A vaccine is now recommended for universal administration to all infants 12 to 23 months of age. The two doses in the series should be administered at least six months apart. A single dose of meningococcal conjugate vaccine (MCV4), a vaccine to prevent sepsis and meningitis, is recommended for 11- to 12-year-olds, for unvaccinated adolescents at high school entry or 15 years of age and for college freshmen who will be living in a dormitory. A single dose of an adolescent preparation of tetanus and diphtheria toxoids and acellular pertussis (Tdap) is recommended for 11- to 12- year-olds, provided they have completed the childhood series and have not yet received the Td booster. Adolescents aged 13-18 years who missed the 11-12 year Td/Tdap booster dose and have completed the childhood series • • • should also receive a single dose of Tdap. The importance of the birth dose of Hepatitis B has been emphasized. Vaccination of infants born to Hepatitis B surface antigennegative mothers can be delayed in rare circumstances, but only if a physician’s order to withhold the vaccine and a copy of the mother’s original HBsAg-negative laboratory report are documented in the infant’s medical record. Influenza vaccine is now recommended for children aged six months and older with certain risk factors, which now specifically include conditions that can compromise respiratory function or handling of respiratory secretions or that can increase the risk of aspiration. The catch-up schedule for persons aged 7-18 years has been changed for Td; Tdap may be substituted for any doses in a primary catchup series or as a booster if age appropriate for Tdap. A five-year interval from the last Td dose is encouraged when Tdap is used as a booster dose. Help Boost Chlamydial Screening Rates Bacterial sexually transmitted diseases, including Chlamydia, are more common than ever. Chlamydia is one of the most widespread STDs in the United States. It is estimated that there are 2.8 million new cases annually. Most infections affect sexually active adolescents and young adults. Chlamydia is usually asymptomatic. It may remain untreated for fairly long periods of time. This can lead to serious complications and transmission of disease to partners. If this condition goes without treatment, it may result in infertility, ectopic pregnancy and chronic pain. The current First Priority Health HEDIS® (Health Plan Employer Data and Information Set) rate indicates Chlamydia screening rates are below optimal levels in females of 16 - 25 years of age (at 15.66%). Improvement in this screening will help to ensure better reproductive health in young women. Prevention, early treatment and screening are vital in battling serious complications and promoting better health. Together, we can make the difference in women’s health. HEDIS® is a registered trademark of the National Committee for Quality Assurance (NCQA). Clinical Practice Guideline Update The following Clinical Practice Guidelines have been updated: 12-8-05: Cholesterol Screening; Cholesterol Management, Secondary Prevention; and Medical Management of Bipolar Disorder During the Maintenance Phase of Treatment in the Outpatient Setting. Practice Guidelines are available on BCNEPA’s Provider Center under the Quality Management Section, accessible via the webbased NaviNet system or via www.bcnepa.com with a secure provider password. If you would like to request a hard copy of the guidelines, please call Ann Marie Wheeler at 570 200-4377, weekdays, 8:00 a.m. to 4:00 p.m. If you would like to obtain a secure provider password, please contact Provider Relations with the following information: Name (first and last); Group or practice name; Type of practitioner (primary vs. specialist); E-mail address or fax address; and Challenge prompt and response (i.e., mother’s maiden name, dog’s name, etc.). This information is required for security reasons.