Soaps and Emulsions - Making soap
advertisement

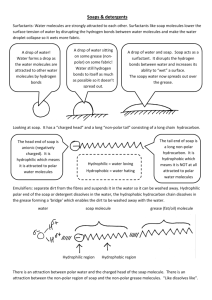
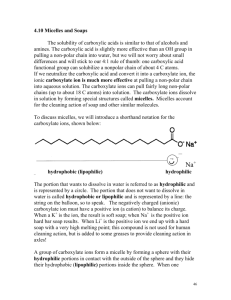
NATIONAL QUALIFICATIONS CURRICULUM SUPPORT Chemistry Soaps and Emulsions Section 1: Making soap [HIGHER] The Scottish Qualifications Authority regularly reviews the arrangements for National Qualifications. Users of all NQ support materials, whether published by Learning and Teaching Scotland or others, are reminded that it is their responsibility to check that the support materials correspond to the requirements of the current arrangements. Acknowledgement Learning and Teaching Scotland gratefully acknowledges this contribution to the National Qualifications support programme for Chemistry. © Learning and Teaching Scotland 2011 This resource may be reproduced in whole or in part for educational purposes by educational establishments in Scotland provided that no profit accrues at any stage. 2 SOAPS AND EMULSIONS (H, CHEMISTRY) © Learning and Teaching Scotland 2011 Introduction The following document has been designed as a guide for practitioners teaching section 7 of the Consumer Chemistry component of Higher Chemistry. This document can be used to explain specific examples to a more in-depth level or to explain general concepts. Section 1: Making soap Section 1: Making soap Making soap Soaps are formed by the alkaline hydrolysis of fats and oils by sodium or potassium hydroxide by boiling under reflux conditions: The glycerol released is separated and used as a raw material for other processes: 4 SOAPS AND EMULSIONS (H, CHEMISTRY) © Learning and Teaching Scotland 2011 Section 1: Making soap The fatty acids are produced in the form of their sodium or potassium salts. These salts are called soap. Soap The long covalent hydrocarbon chain that makes up the tail section of a soap structure can be represented in a number of ways, either in the shorthand notation shown below or as a bond-stick representation, shown at the bottom of the page. The charged carboxylate group represents the head section of the soap structure. Soap Section 1: Making soap The structure of soap The long covalent hydrocarbon chain gives rise to the hydrophobic (water hating) and oil-soluble (non-polar) properties of the soap molecule (represented in yellow). The charged carboxylate group (represented in blue) is attracted to water molecules (hydrophilic) . In this way, soaps are composed of a hydrophilic head and a hydrophobic tail: How soaps work The following ball (blue for hydrophilic head group) and stick (yellow for hydrophobic tail group) diagram represents the initial interaction of soap on addition to water and material with a grease stain: 6 SOAPS AND EMULSIONS (H, CHEMISTRY) © Learning and Teaching Scotland 2011 Section 1: Making soap When the solution containing soap and water is agitated (stirred vigorously) the interactions of hydrophobicity and hydrophilicity become apparent. The hydrophobic, non-polar, tails burrow into the greasy, non -polar molecule – like attracting like. In the same way the polar hydrophilic head groups are attracted to polar water molecules. The head groups all point up into the water at the top of the grease stain. The attraction of the head group to the surrounding water, via polar-to-polar interactions, is so strong that it causes mechanical lift of the grease molecule away from the material on which it was deposited. The hydrophobic tails are anchored into the grease due to non-polar to non-polar attraction. In combination, these effects allow for the removal of the grease stain.