CH437 CLASS 19
advertisement
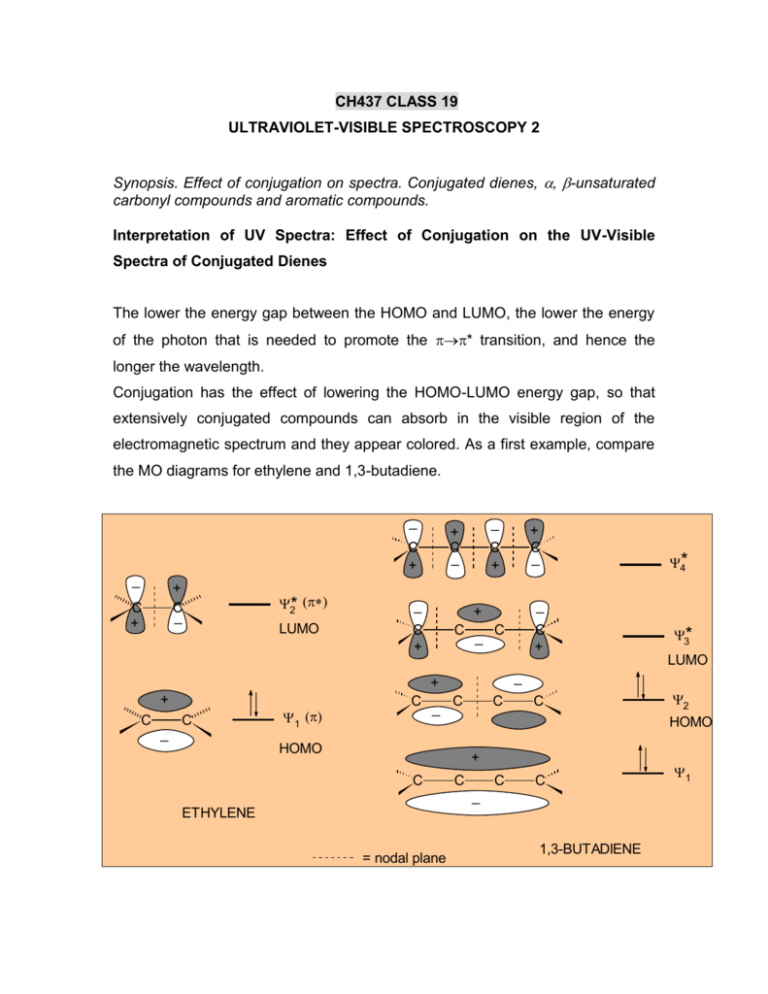
CH437 CLASS 19 ULTRAVIOLET-VISIBLE SPECTROSCOPY 2 Synopsis. Effect of conjugation on spectra. Conjugated dienes, , -unsaturated carbonyl compounds and aromatic compounds. Interpretation of UV Spectra: Effect of Conjugation on the UV-Visible Spectra of Conjugated Dienes The lower the energy gap between the HOMO and LUMO, the lower the energy of the photon that is needed to promote the * transition, and hence the longer the wavelength. Conjugation has the effect of lowering the HOMO-LUMO energy gap, so that extensively conjugated compounds can absorb in the visible region of the electromagnetic spectrum and they appear colored. As a first example, compare the MO diagrams for ethylene and 1,3-butadiene. _ C + _ + C _ C + 2* LUMO _ + C _ _ C + C + C _ C C _ 1 C _ C C + C C C LUMO 2 HOMO + C C C _ ETHYLENE = nodal plane 3* _ HOMO C 4* _ + + + + C _ 1,3-BUTADIENE 1 The most important transition is the HOMO-LUMO transition (* for ethylene and 2*3 for 1,3-butadiene). This corresponds to (max ~175 nm for ethylene and 217 nm for 1,3-butadiene, so clearly conjugation closes the HOMO-LUMO energy gap and increase max (bathochromic shift), as shown below This gives rise to the UV spectrum below, where absorbance is plotted against wavelength (). Taking the argument further, compare 1,3-butadiene (max = 217 nm) and 1,3,5hexatriene (max = 258 nm). From this UV spectral data, we can calculate the HOMO-LUMO energy gap as 553 kJ/mol and 465 kJ/mol for 1,3-butadiene and 1,3,5-hexatriene, respectively. The spectra below show the HOMO-LUMO (* type) transitions for conjugated dienes with up to five double bonds. Colored Organic Compounds Extension of conjugation can lead to such a low HOMO-LUMO energy gap that the molecule absorbs in visible region of the electromagnetic spectrum and the compound appears colored. Such an example is -carotene, with 11 conjugated double bonds: its UV/visible spectrum and structure are shown below. When normal (“white”) light strikes –carotene, the wavelengths from 400 nm to 500 nm are absorbed, corresponding to green – blue, while other wavelengths are reflected or transmitted to our eyes. We see white light with green-blue removed and hence we perceive a yellow-orange color for –carotene. Effect of Substituents For conjugated systems, just as extension of conjugation influences max, so does the presence of substituents (auxochromes). This occurs through interaction of the main chromophore -system with either the non-bonded electrons of a heteroatom (conjugation) or the electrons bonded in a C-H group (hyperconjugation). Either way, the net result is an extension of the chromophore conjugated -system, and a concurrent decrease in the HOMO-LUMO energy gap, as shown below. CONJUGATION _ + C _ C + 2 + C C 3 * _ + n _B 1 C C .. C C B .. B + or B _ HYPERCONJUGATION H + + + + C C _ _ C _ H H UV-Visible Spectra of , -Unsaturated Carbonyl Compounds Two principal transitions are associated with the carbonyl group, the weak (forbidden) n* and the strong (allowed) * transitions, but the latter is below the cutoff points of solvents: * n ~280 nm (weak) 190 nm (strong) Substitution on the carbonyl group by an auxochrome with a lone pair of electrons (e.g. –NR2, -OR, NH2, -OH, or -X as in amides, esters, acids or acid halides) leads to a hypsochromic effect on the n* transition and a lesser bathochromic shift on the * transition, but latter nearly always remains below the solvent cutoff wavelength. The hypsochromic effect, due to inductive (-I) effects of the more highly electronegative nitrogen, oxygen or halogen atom, is illustrated for the n* transition of the acetyl (CH3-C=O) group, below. If the carbonyl group is part of a conjugated system, both the n* and the * are shifted to longer wavelengths (bathochromic shifts) and the latter becomes much more intense (hyperchromic shift). The former transition eventually becomes hidden by the latter, as illustrated below, showing the UV-visible spectra of conjugated aldehydes (CH3-(CH=CH)n-CHO). The diagram below (not drawn to scale) illustrates the influence of conjugation on the molecular oribital energies and corresponding changes in the wavelengths of the n* and particularly the * transition. Substituents and other features influence the * transition of the basic chromophore in a predictable way. A study of these led to the formulation of empirical rules for ,-unsaturated carbonyl compounds by Woodward and Fieser (Class 20). UV-Visible Spectra of Aromatic Compounds Absorptions that result from transitions within the benzene chromophore are more complex than the MO system would suggest: three absorption bands are actually observed. These are at max ~ 184 nm (max ~ 47,000) (allowed), max ~ 202 nm (max ~ 7,400) (forbidden) and max ~ 255 nm (max ~ 230) (forbidden). The MO system of benzene is shown below. Symmetry effects, combined with electron-electron repulsions, result in modification of the energy levels (from the MO energy level diagram above) that are involved in the UV absorption spectrum of benzene. They are basically * transitions: E 1u B 1u 200 nm (forbidden) 255 nm (forbidden) B 2u 180 nm (allowed) A 1g The A1gB2u transition gives rise to the low intensity “secondary band” at ~255 nm and the A1gB1u transition results in the higher intensity “primary band” at ~ 202 nm in benzene. Both these transitions are influenced by substituents on the benzene ring. Electron releasing groups (donors) increase the max value of both primary and secondary bands (red shift or bathochromic shift), as well as max (hyperchromic shift). Electron withdrawing groups have the same effect on max and max of the primary band, but effects on the secondary band are less pronounced. Influence on the primary band transition is caused by extension of the delocalized by aromatic -system because of interaction with molecular orbitals on the substituent group: ..+ OCH3 .. : OCH3 .. O: H H C C _: .. _ : O .. + etc etc Influence of substituents is summarized in the table below. Substituent Primary Secondary max/nm max/cm l mol-1 max/nm max/cm l mol-1 H 203.5 7400 254 204 CH3 206.5 7000 261 225 Cl 209.5 7400 263.5 190 Br 210 7900 261 192 OH 210.5 6200 270 1450 O- 235 9400 287 2600 OCH3 217 6400 269 1480 NH2 230 8600 280 1430 NH3+ 203 7500 254 169 CN 224 13000 271 1000 CO2H 230 11600 273 970 CO2- 224 8700 268 560 COCH3 245.5 9800 CHO 249.5 11400 NO2 268.5 7800 The spectra (max) of substituted benzoyl derivatives can be predicted by the use of Scott’s empirical rules (see Class 20). Both the primary and secondary bands in the spectra of polynucear aromatic hydrocarbons are shifted to higher wavelength – even the lower wavelength primary band is shifted to wavelengths over 200 nm, readily accessible by standard UV instruments. The spectra of naphthalene and anthracene are shown below.







