quant7LimReagS`more
advertisement

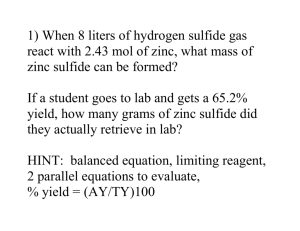
from yesterday's demo: ex. Haber process N2 + 3H2 2NH3 recall mole ratio: the relationship between moles in a chemical equation, shown by the coefficients of a balanced chemical equation in this case for reactant 1:3 nitrogen gas to hydrogen gas let's say I have stoichiometric amounts of each reactant, how much of the products would I produce? 2 moles of nitrogen gas and unlimited H2? what would happen if we had 2 moles of nitrogen gas, but only 3 mole of hydrogen gas? - in nature you usually don't have stoichiometric amounts of reactants, ex. cellular respiration C6H12O6 (s) + 6 O2 (g) 6 CO2 (g) + 6 H2O (l) - what do you think will be most likely to stop this reaction from happening? LIMITING AND EXCESS REAGENTS limiting reagent - reactant that is completely used up in the reaction - determines how much product will be produced excess reagent - reactant that remains after the reaction is over ex. If 13.7 g of butane, C4H10 is combusted in 14.2 g of oxygen gas to produce carbon dioxide and water find: a. b. c. d. the limiting reagent the mass of carbon dioxide produced the mass of water produced. the mass of excess reagent which remains unreacted - write balanced chem. equation 2 C4H10(g) + 13 O2(g) 8 CO2(g) + 10 H2O(l) - write mole ratio - convert masses to moles - calculate amount of product produced for both butane and O2 - whatever is lower is your limiting reagent balanced rxn 2 C4H10 13 O2 8 CO2 10 H2O mole ratio 2 13 8 10 mass (g) 13.7 14.2 M (g/mol) sldkfjsdofk 32.00 n (mol) ex. In the textile industry, chlorine gas is used to bleach fabrics. Any of the toxic chlorine that remains after the bleaching process is destroyed by reacting it with a sodium thiosulphate solution: Na2S2O3 (aq) + 4 Cl2 (g) + 5 H2O (l) 2 NaHSO4 (aq) + 8 HCl (aq) If 135 kg of sodium thiosuphate reacts with 50.0 kg of Cl2 and 238 kg of water how many grams of sodium bisulphate are expected? balanced rxn mole ratio mass (g) M (g/mol) n (mol) Limiting Reagent: S'mores Lab S'mores are a Canadian camping delicacy – a treat made over an open fire at the end of an exhausting day spent hiking, canoeing, and generally having way way too much fun without a TV around. Today, we will use a microwave to make s'mores. The balanced reaction for making s'mores is as follows: 2 Gc + 3 Cc + 1 Mm 1 S'more where Gc is a Graham cracker, Cc is a chocolate chip, and Mm is a marshmallow. 1. Using your reagents (provided by the teacher), and the balanced S'mores reaction, calculate whether you have too few Gc or Mm to carry out a reaction that will give you 2 S'mores. Cc is your excess reagent. Gc from balanced equation, what is your mole ratio? how many moles of each reactant do you have? how many moles of each reactant do you need to make 2 mol of S'mores? Cc Mm S'more excess excess 2 2. What are you missing? How many? This is your limiting reagent. limiting reagent: excess reagent: 3. Using this lab and your textbook, define excess and limiting reagents (pp.187-188). excess reagent: limiting reagent: 4. Once the above questions are completed bring this sheet to the teacher to obtain enough Gc or Mm to produce 2 S'mores. Bon appetit! *by Bogna Haddad, adapted from Donna, Dept. of Science, Notre Dame High School. portfolio: LIMITING REAGENTS 1. If 3.85 g of concentrated sulphuric acid and 2.927 g of sodium chloride are mixed to produce sodium sulphate and hydrochloric acid find: H2SO4 + 2 NaCl a. b. c. d. the the the the Na2SO4 + 2 HCl limiting reactant. mass of hydrogen chloride produced mass of sodium sulphate produced. mass of excess reagent which remains unreacted. (Ans. 1.39 g) 2. If 4.57 g of hydrochloric acid is added to 5.45 g of zinc to produce zinc chloride and hydrogen gas find: Zn(s) + 2 HCl(aq) a. b. c. d. the the the the ZnCl2(aq) + H2(g) limiting reactant. mass of hydrogen gas produced mass of zinc chloride produced. mass of excess reagent which remains unreacted. (Ans. 1.35 g) 3. If 27.2 g of gaseous ammonia is passed over 135.6 g of heated copper(II) oxide to produce nitrogen, copper and water vapour find: 2 NH3(g) + 3CuO(s) a. b. c. d. e. the the the the the N2(g) + 3Cu(s) + 3 H2O(g) limiting reactant. mass of nitrogen formed mass of copper formed. mass of water formed mass of excess reagent which remains unreacted. (Ans. 7.85 g)