A Description of Nutrient Variability within Trees in Northern
advertisement

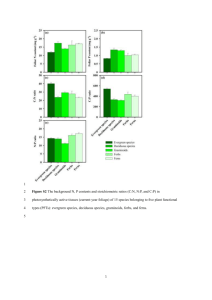
YANG | 2013 A Description of Nutrient Variability within Trees in Northern Hardwood Forest Yang Yang A Final Report for Edna Bailey Sussman Internship December 2013 Introduction and Immediate Objectives Nutrient concentrations in trees are usually considered to change over time due to the nitrogen deposition, acidification or harvesting. However, the base cation losses from soil caused by harvesting (Lamontagne et al., 2000) and acid rain (Fernandez et al., 2003) may pose a potential threat to forest health and productivity, impairing the sustainability of forests in the long-term. Addressing the change of nutrient concentrations in tree tissues over time can help to recalculate the nutrient budget for accessing forest sustainability and make it possible to compare across distinct sites with statistical confidence. At Hubbard Brook Experimental Forest (HBEF), a Long-Term Ecological Research site in New Hampshire, nutrient concentrations in tree tissues were reported in 1960’s but have not been monitored over time. To detect the change of tree nutrients over time requires repeated measurement. However, the methods for collecting those samples were not fully documented. This proposed project will describe how variable the nutrient concentrations are within trees due to the different tissue positions, which makes it possible to determine whether differences in tree nutrients observed between 1965 and near future could be due to uncertainty in the early sampling methods. During my internship period under Edna Bailey Sussman Fellowship, I discussed with my intern advisor Dr. Richard Hallett of the USDA Forest Service, my major professor Dr. Ruth Yanai of SUNY-ESF and site manager Ian Halm of the HBEF, and wrote a method proposal approved by the Research Approval Committee (RAC) in HBEF. The immediate objective for 1 YANG | 2013 this project is to remove the barriers of vague sampling methods for detecting change over time in tree chemistry. Work Completed Site description To address the uncertainty of detecting change in tree nutrients over time, trees for sampling were fell down for dissecting in the watershed 7, the “no treatment design” watershed of the HBEF (43°56′N, 71°45′W). Hubbard Brook Experimental Forest (HBEF), located in the White Mountain National Forest in central New Hampshire, is dominated by American beech (Fagus grandifolia Ehrh.), sugar maple (Acer saccharum Marsh) and yellow birch (Betula allegheniensis Britt.). Climate there has an average temperature of -9℃ in January and 18℃ in July with an annual precipitation at 130 cm (Likens et al., 1977). Soil is mainly well-drained Spodosol developed in glacial till (Huntington et al., 1988). Sampling method One vigorous tree of three species (American beech, sugar maple and yellow birch) with DBH over 25 cm was selected and cut down uphill in the woods in the watershed 7 in HBEF. Length of the tree crown Middle crown Upper crown Lower crown 1/2 1/2 Disc Disc Disc 9 cm 1/3 1/3 1/3 Fig 1: Sampling strategy for foliage and disc samples from fell down tree 2 YANG | 2013 Figure 1 illustrates the sampling position for foliage and disc samples. The diameter of each tree was measured at 1.3 meters above the ground. The total height of tree and the length of tree crown were measured after trees being fell down. The base of the live crown was defined as the height of the foliage in the lowest branch (The distance of the lowest branch to the second lowest branch should be within 2 inches). Within these trees, health foliage without petioles from 3 canopy positions (figure 1), branch samples from five diameter classes (0.5 cm, 1 cm, 2 cm, 3 cm, over 3 cm) were collected in the up side of tree which was more collectable. Three logs were cut evenly from the base of the tree to up to the merchantable bole (9 cm diameter). 5 cm disc was dissected out from the bottom of each log (Figure 1). Chemical analysis Nine discs collected from fell down trees in total were dissected into bark, sapwood and heartwood quickly after bringing back to the lab. The edges of disc had been touched with chainsaw were trimmed away first, and the wood samples from the discs were cut down into small pieces using a clean chisel. Bark samples from both sites were examined and washed in a detergent solution (1% Alconox) if extra materials like lichens or algae exist, and then rinsed three times in deionized water (Likens and Bormann, 1970). All the foliage, branch, bark and wood samples from two sites were dried at 60 ℃ in the oven. After being dried, tissue samples were ground in a Wiley mill to pass a 20 mesh screen. Total N was analyzed using a carbon-nitrogen elemental analyzer. Subsamples were ashed at 470℃ and dissolved in 10 mL of 6M HNO3 on a hot plate (Siccama et al., 1994). Concentrations of P, K, Ca and Mg from the acid solution were determined by an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES). Drift of less than 5% was allowed during the ICP processing. Apple leaf (Philenoptera violacea) was used as a standard reference material to test the methods of analysis for plant tissue. Observed mean N concentration was 22.73 ± 0.05 mg/g (Certified value 22.50 mg/g), mean P concentration was 1.54 ± 0.03 mg/g (Certified value 1.59 mg/g), K concentration was 15.59 ± 0.50 mg/g (Certified value 16.10 mg/g), Ca concentration was 15.36 ± 0.60 mg/g (Certified value 15.26 mg/g) and Mg concentration was 2.70 ± 0.05 mg/g (Certified value 2.71 mg/g). 3 YANG | 2013 Preliminary Results and Future Work Figure 2: Nutrient concentrations in foliage by canopy. Data average the three species. Concentrations of four nutrient elements showed a decreasing trend in foliage through bottom canopy to top canopy. The differences of concentrations of K and Ca by canopy are bigger than those of P and Mg (Figure 2). K is usually considered to be washed out from foliage by rains quickly which makes the bottom canopy showed a higher concentration in K. The foliage in top canopy is most likely the sun-leave which has more chances of processing photosynthesis, causing a higher usage of Ca in foliage. K×2 Figure 3: Concentration of K (times 2) in parts per thousand in branch by branch diameter. Data average the three species. 4 YANG | 2013 In general, concentration of K in branch (branch wood + branch bark) showed a decreasing pattern because of the increasing ratio of wood to bark mass. Concentration of K dropped quickly when the branch diameter increased from 5 mm to 10 mm, and began to decrease slowly. When the branch diameter reached 30 mm, there was less K concentration in the branch (Figure 3). Based on the two preliminary results mentioned above, we might ignore the canopy level and only sample the bottom foliage which is more reachable if we only study the changes in concentrations of P and Mg in foliage over time. For studying the K concentration in branch, branch less than 10 mm or larger than 20 mm should be counted for knowing the specific numbers of branches since the K concentration begins to vary when the branch diameter is in that range. Future work would be completing to quantify the variability of nutrient concentrations (N, P, K, Ca and Mg) within trees for different tissue types. Taking advantage of my thesis project, for which I re-sampled the trees with same species in Huntington Wildlife Forest (NY) in 2010s based on the sampling documents from 1980s, I will analyze the changes in nutrient concentrations over time with reference to the uncertainty in sampling methods that I quantify here. This will give us some suggestions and guidance when we begin to conduct a huge project of monitoring tree nutrients in HBEF in the near future. Acknowledgements I would like to thank my advisor, Ruth Yanai and Richard Hallett for helping design the experiment. Few scientists also gave me comments and suggestions on my project: Tim Fahey (Cornell), Russell Briggs (SUNY-ESF), Scott Bailey (USFS) and John Battles (UC-Berkeley). I also want to thank the site manager of HBEF, Ian Halm for assisting me to do the field work with safety guidance. Field assistance was provided by my shoestring members in Multiple Element Limitation in Northern Hardwood Ecosystems (MELNHE) project: Yi Dong and Hongzhang Kang. Laboratory assistance was provided by Debra Driscoll and Chuck Schirmer at SUNY-ESF. The Edna Bailey Sussman Foundation gave me the generous support, and I will continue to acknowledge it in all the presentations and publications related to this project. 5