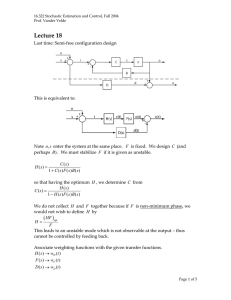
Retroviral Infection and Generation of Stable Cell Lines- To
advertisement

Supplementary Methods Retroviral Infection and Generation of Stable Cell Lines- To generate Smad3 expressing retroviruses, Pheonix E packaging cells were plated at 106 cells/60-mm-diameter tissue culture dishes and transfected with the LPCX retroviral vector (empty or containing Flag-WT Smad3) by the calcium phosphate method. In the case of TRII expressing retrovirus, AVOSC packaging cells were transfected with the MFG-CAT retroviral vector (empty or containing TRII). At 6 h posttransfection, the medium was replaced with fresh DMEM containing 10% FBS, and cells were grown for an additional 24 h. The conditioned medium containing recombinant retroviruses was collected and filtered through 0.45 m-pore-size polysulfonic filters. Samples of these supernatants were applied immediately to SNU484 cells or SNU638 cells, which had been plated 18 h before infection at a density of 105 cells/60-mm-diameter tissue culture dishes. Polybrene (Sigma, St. Louis, MO, USA) was added to a final concentration of 8 g/ml, and the supernatants were incubated with the cells for 12 h. After infection, the cells were placed in fresh growth medium and cultured as usual. Selections with 1 g of puromycin/ml or 400 g of neomycin/ml were initiated 24 h after infection. After about 15 days, individual clones picked from plates of the recombinant retrovirus-infected cells were transferred to 24 microtiter wells and expanded to generate cell clones stably expressing Smad3 and TRII. Immunohistochemical Staining- Tissues were fixed in 10% neutral buffered formalin, mounted in paraffin, and 5 m sections were deparaffinized. Endogenous peroxidase was blocked with 3% H2O2 in methanol for 30 min. Non-specific protein binding was blocked with a solution containing 1% bovine serum albumin (BSA) and 5% goat serum for 30 min. Sections were incubated for 2hr with the primary antibodies anti-Smad3 (I-20; Santa Cruz, CA, USA), anti-CEA (A0115) in TBS/1% BSA. Rabbit IgG at 4 g/ml was used as a negative control. Antigen-antibody complexes were detected using the Vectastatin Elite avidin biotin complex (ABC) peroxidase kit from Vector Laboratories (Burlingame, CA, USA) according to the manufacturer’s instructions. After a 30-min incubation with ABC reagent, a 5-min reaction with 3,3’-diamino benzidine/H2O2 (BioGenex, San Ramon, CA, USA) was used to detect the bound peroxidase. Slides were counterstained with Carazzi haematoxylin. Isolation of RNA and RT-PCR- Total RNA was isolated from human gastric cancer cells using Trizol (Gibco BRL, Gaithersburg, MD, USA). RT-PCR was performed according to the manufacturer’s instruction using a TITANIUMTMOne-Step RT-PCR kit (BD Biosciences Clontech, Palo Alto, CA, USA). The primers used were as follows: CEA (CEACAM5) (sense/anti-sense), 5’GAAATGACACAGCAAGCTAC-3’/ 5’-GGACAGCTGCAGCCTGGGAC TGAC-3’ (product length: 496 bp), CEACAM6 (sense/antisense), 5’-GTCACAGCAGCCCTGACCA GAGC-3’/5’- GTTGCTTCTTCATTCACAAGATCTG-3’ (product length: 481 bp), CEACAM8 (sense/antisense), 5’-GACAGCACAGCTGACAGCCGTGC-3’/ 5’-CCAGTTGTAGCCACGAGGGTC-3’, (product length: 286 bp) and human glyceraldehydes-3-phosphate dehydrogenase (GAPDH) (sense/antisense), 5’-GCAGGGGGGAGCCAAAAGGG-3’/ 5’-TGCCAGCCCCAGC GTCAAAG-3’ (product length: 566 bp). PCR was performed with a 20 l volume. The amplification cycle (denaturation step at 94C for 30 sec, and annealing step at 65.0C for 30 sec, and an extension step at 68C for 1 min) was repeated 30 times and followed by a final extension for 2 minutes at 68C. Northern Blot Analysis- Ploy(A)+ RNAs of human gastric cancer cells were isolated with Trizol. RNA was electrophoresed on 1.0% agarose-formaldehyde gel and transferred to nylon membrane, and cross-linked using UV Stratalinker (Stratagene, La Jolla, CA, USA). Blots were hybridized in Rapid-hyb buffer (Amersham Pharmacia Biotech, Piscataway, NJ, USA) at 65C for 20 h. 32 P- labeled CEACAM1, CEA and CEACAM6 probes, generously provided by Dr. C. P. Stanners (McGill University, Canada) and 1.5-kb fragment of human TRII gene were prepared using PRIME-IT random primer labeling kit (Stratagene). Generation of mice and histological examination- Smad3ex8/ex8 mice were generated by targeted disruption of the Smad3 gene by homologous recombination. Targeted embryonic stemcell clones were microinjected into C57BL/6 blastocysts to obtain germline transmission. Mice heterozygous for the targeted distruption were intercrossed to produce homozygous offspring (Yang et al., 1999). All animals were killed under deep CO2 anethesia at the 10th week from the birth date and laparotomized with excision of their stomachs. The stomachs were opened along the greater curvature and only the glandular stomach was harvested. Tissues were fixed in 10% neutral formalin in PBS, processed by standard method, and embedded in paraffin. Tissues were sectioned at 4 m for histological examinations.