Whole mount In Situ Hybridization
advertisement

Whole mount in-situ Based on the Ronna’s protocol
Whole mount In Situ Hybridization
I. In vitro transcription using DIG labeling (Mia)
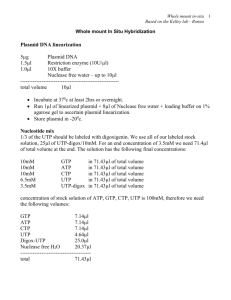
Plasmid linearization
Linearization: 20g DNA restricted with the appropriate enzyme (50U) in 100ul
Incubate 2h / O.N and run on agarose gel to see full digestion
Inactivation
Remember to plan restriction of antisense for the probe and sense for the control
Use 5’ protruding termini or blunt.
Extract DNA
Add 100l of RNase free water
Add 1 volume (200l) of chloroform/phenol 1:1 to the restricted DNA 1
and mix well
Spin 5 min X 2,000rpm
Remove supernatant to a new tube
Optional – add 200l chloroform, mix well and spin again
Remove supernatant to a new tube
Add 20l (10% from total volume) 3MNaAC (pH=5, DEPC treated)
Add 400l (2 volumes) 100%ice cold EtOH - mix
15’X dry ice in EtOH bath
Spin 10’X4c X max speed
Remove EtOH and add 800l 70% ice cold EtOH
10’X dry ice in EtOH bath
Spin 10’X4c X max speed
Resuspend to 1g/l in ddw (DEPC-treated or better with Nuclease free water)
Run 0.5l on 1% agarose gel
Dig cRNA labeling
Mix the reagents in the following order at R.T:
RNase free water
10X transcription buffer
0.1M DTT
Nucleotide mix
RNase inhibitors
RNA polymerase (10U/l)
Add 2l (2g) of linearized DNA template
23l
4l
4l
4l
1l
2l
Incubate 2hX 37c
1
Check the phenol color – shouldn’t be brown (EZ-RNA kit has phenol-chloroforme solution)
1
Whole mount in-situ Based on the Ronna’s protocol
Take 0.5l for gel check. The RNA band should be ten fold brighter than the DNA band
DNase treatment
2l DNase I RNase free
1l RNase inhibitors (20U)
Incubate 15-30’ X 37c
Inactivation 10’ X 65c
RNA purification
Add RNase free water
- 180 l or
RNase free water 100l
add 10% volume 3M NaAC (in DEPC) - 20 l
4M LiCl
4l - mix
add 3 volume of ice cold 100% EtOH - 600 l
100%EtOH
300l
mix well & place in -20c X 30’ (or dry ice)
* Can precipitate O.N.
Centrifuge 12,000 rpm X 4c X 10’
Wash with 600l of 70% ice cold EtOH
Remove supernatant – air dry & resuspend pellet with 40l RNase free water
Run 0.5l on 1% agarose gel and photocopy immediately
Store plasmid in -200c / aliquots in -70c.
- Alternatively; cleaning on G-50 column or by gel extraction if more than 1 band is seen
Check the phenol color – shouldn’t be brown (EZ-RNA kit has phenol-chloroforme solution)
II. Tissue Prep for ISH
A. Dissection
1. Dissect embryos in PBS (remove membrane as much as possible)
Use tools treated with RNase away and disposable bulbs
From E9.5 punctate mylencephalon and make small hole in the heart and telencephalon
2. Fixation in 10 ml fresh 4% paraformaldehyde at 4c X O.N X gentle rocking
B. Dehydration ~1h
24 hours after fixation
Use 50 ml tube with 20 ml solution of:
PBT X 5’X 4c
-X2
25% methanol in PBT 5’X R.T
50% methanol in PBT 5’X R.T
75% methanol in PBT 5’X R.T
100% methanol
5’X R.T - X 2
Can store at -20c as long as a month
2
Whole mount in-situ Based on the Ronna’s protocol
3
C. Rehydration ~1h (in the experiment day)
Use 50 ml tube with 50 ml solution of (diluted with in PBT):
75% methanol in PBT 5’X R.T
50% methanol in PBT 5’X R.T
25% methanol in PBT 5’X R.T
PBT X 5’X 4c
-X2
III. Pre-treatments/Deproteination
~1h40min (Ronna)
1. Treat with 10 µg/ml proteinase K in PTW for 10 min2
2. Wash 30 sec in 2 mg/ml glycine in PBT (fresh) X R.T
Wash 10’ in 2 mg/ml glycine in PBT (fresh) X R.T
3. Carefully rinse with PTW 2X 5 min
4. Incubate in Detergent Mix 2X 15 min, then rinse 2X 5 min PTW
5. Post-fix in 4% PFA+0.2% Glutraldehyde for 20 min
6. Rinse with PTW x 5 x shaker
IV. Hybridization – 1h30min until the o/n incubation
1. Rinse in 1:1 PTW to hybridization mix and let cochleae settle
2. Rinse in 1 ml hybrid mix and let cochleae settle
3. Incubate horizontally at 65ºC on shaker in 1ml fresh hyb mix for 1 hour
4. Add 1ml fresh pre-warmed hyb mix and 1µg (varies) DIG-labeled probe
5. Incubate horizontally at 65ºC on shaker overnight (O/N)
---------------------------------------------End of day #1-------------------------------------------------------V. Post-Hybridization Washes
1. Rinse 2X in pre-warmed hyb mix
2. Wash 2X 30 min at 65ºC with 1.5ml pre-warmed hyb mix
3. Wash 10 min at 65ºC with 1:1 hyb mix to MABT pre-warmed
4. Rinse 2X with 1.5ml MABT x 5’ x 65ºC
5. Wash 15 min in 1.5ml MABT x 65ºC
**optional: incubate 1 hr with 1.5 ml MABT and 2% Boehringer Blocking Reagent (BBR), then add 2%
BBR to step 6 and step 7.
6. Incubate 1 hour in MABT with 20% heat-treated sheep serum horizontally at 65ºC on shaker
7. Incubate 3 hours RT or O/N at 4ºC on shaker in MABT, 20% sheep serum, and 1/1000 dilution APanti-DIG antibody
---------------------------------------------End of day #2-------------------------------------------------------VI. Post-Antibody Washes
1. Rinse 3X with MABT x 5’ x R.T x shaker
-Transfer tissue into glass vials
2
Our proteinase K is at a 15mg/ml concentration. Add 0.7µl to 1ml of PTW.
Whole mount in-situ Based on the Ronna’s protocol
2. Wash 2X 45 min with MABT at Room Temperature (RT) while shaking horizontally
VII. Histochemistry
1. Wash 2X for 10 min in 5ml NTMT (fresh)
2. Incubate at RT in the dark while shaking with NTMT-BCIP-NBT mix
* purple color will develop (10 min- >3 days)
*change solution every few hours until color develops
3. Remove solution when color is developed
4. Wash for 10 min with 5ml NTMT
5. Wash for 10 min with PBS
*can be stored in PBS at 4ºC
VIII. Bleaching (Optional)
-Used to minimize background color
1. Warm cochleae to RT if originally at 4ºC
2. Replace PBS with 70% or 90% EtOH
3. Watch closely under microscope for background to start fading (about 3 min)
4. When desired color is reached, quickly remove EtOH and replace with PBS
Solutions:
4% Paraformaldehyde in PBS
It’s better to use fresh PFA up to 1 week is O.K
Remember to put all leftovers in organic waste
To make 250ml of 4% Paraformaldehyde
Wear disposable gloves throughout the procedure
1. Use a sterile 250ml Erlenmeyer flask.
2. Weigh out 10g of paraformaldehyde powder into flask.
3. Using disposable 50ml centrifuge tube, measure out 40ml of sterile water and put in flask.
4. Heat to 650c in an H2O bath.
5. Once solution has warmed to 650c, add 50µl of 10N NaOH, using a P200 tip.
6. Continue to swirl and heat solution in H2O bath.
7. Add 25ml of 10X PBS (Phosphate Buffer Saline), and 80µl of 6M HCl.
8. Bring solution up to 250ml with sterile H2O.
9. Check pH with litmus paper. Should range from 7.2 to 7.4.
10. Filter solution with 250ml filter bottle.
11. Label and place in cold room until ready to use.
PTW: 0.1% Tween-20 in PBS (1X)
Detergent Mix:
IGEPAL
SDS
deoxycholate
1 M Tris-HCl pH8
500 mM EDTA pH8
5M NaCl
500 µl
5 ml
0.25 g
2.5 ml
100µl
1.5 ml
4
Whole mount in-situ Based on the Ronna’s protocol
DEPC H20
40.4 ml
-------------------------------------------------50.0 ml
Hybridization Mix: Formamide
25.00 ml
SSC (20x pH5 w/citric acid)3 3.25 ml
EDTA (0.5 M pH8)
0.50 ml
Yeast RNA4 (5mg/ml)
0.50 ml {final conc 50 µg/ml}
Tween-20 (10%)
1.00 ml
CHAPS (10%)
2.50 ml
Heparin (50 mg/ml)
0.10 ml {final conc 100 µg/ml}
Water
17.15 ml
----------------------------------------------------Total
50.00 ml
*Store at -20°C
5X MABT:
Maleic Acid (50mM)
11.6 g
5
NaCl (0.74M )
8.7 g
Tween-20
11.0 ml
Water
185.0 ml
--------------------------------------------------Total
200.0 ml
Mix Malex acid in ddw
Adjust pH to 7 using NaOH
Add NaCl (8.7g or 30 ml from 5M solution)
NTMT:
5M NaCl
2.0 ml
1M TrisHCl
10.0 ml
2M MgCl2
2.5 ml
10% Tween-20
10.0 ml
Water
75.5 ml
-------------------------------------------------Total
100.0 ml
NTMT-BCIP-NBT Mix:
NTMT
10 ml
BCIP(50 mg/ml in NTMT) 26.2 µl
NBT (75 mg/ml in DMF*) 33.7 µl
*Dimethyl Formamide
Sheep serum (CHEMICON S22-100mL)
Inactivate 56c X 30’
3
250ml 20SSC pH7 + 50ml 1M citric acid. For a 1M citric acid solution – take 29.4gr in 100ml of nuclease free water.
Other protocols use baker yeast tRNA at a 1mg/ml final concentration.
5
Take 30ml of a 5M solution.
4
5
Whole mount in-situ Based on the Ronna’s protocol
Cool on ice
Centrifuge and dived the sup to 1 ml aliquots (important in order to lose the protein precipitate)
BBR (Rochecat. No. 1 096 176)
Prepare X10 stock solution
Dissolve blocking reagent in Maleic acid buffer to final concentration of 10% (w/v)
Mix and heat to 60c X 1h (do not boil) and make sure reagent is completely dissolved
Store in aliquots of 1.5 ml in –15 to -25c
Dilute the X10 solution with Maleic acid buffer to a X1 blocking solution on the experiment day.
Always prepare fresh
Maleic acid buffer:
100mM Maleic acid
250mM NaCl
adjust pH to 7.5 using conc. Or solid NaOH
6