Stability Graph
advertisement

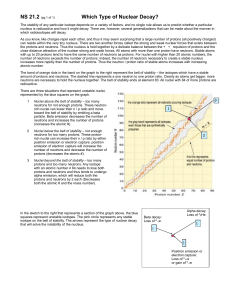
Stability Graph Chlorine atoms have 17 electrons. This is the same as the atomic number of Chlorine. Chlorine atoms can have different numbers of neutrons. Chlorine 35 (atomic mass) has 18 neutrons. Chlorine 37 has 20 neutrons. Cl 35 and Cl 37 are called isotopes of Chlorine. If the n/p (neutron/proton) ratio is far from one, the atom will be radioactive and decompose. Procedure: 1. Complete the data table. 2. Graph the elements, X-axis protons, Y-axis neutrons. Create a scale that uses the whole graph paper. Connect the points in a smooth curve. Data: Element Symbol Boron 11 Oxygen 16 Magnesium 24 Silicon 28 Chlorine 35 Calcium 40 Chromium 52 Cobalt 59 Zinc 65 Bromine 80 Strontium 87 Molybdenum 96 Silver 108 Tin 119 Iodine 127 Barium 137 Tungsten 184 Iridium 190 Gold 197 Argon 40 Neutrons Protons Ratio n/p Stability Graph Part II 1. Graph the following isotopes in a different color on the same graph from part I. Use your graph to decide which of the following elements will be radioactive. If they are, determine which particle it will emit and write the nuclear equation for the decay in the following data table. Nuclei of elements that lie to the left of the band of stability & have a ratio 1 or (1.5 if atomic number greater than 20) have too many neutrons and will usually decay by emitting Beta particles. Nuclei of elements that lie to the right of the band of stability & have a ratio 1 or (1.5 if atomic number greater than 20) have too many protons and will decay by electron capture or positron emission. Nuclei of elements that are above the band of stability or have an atomic number greater than 83 have too many protons and neutrons and will decay by emitting alpha particles. Isotope Symbol Neutrons Protons Ratio n/p Is it Radioactive Type of Emission Nuclear Equation Carbon 14 Polonium 210 Nitrogen 14 Magnesium 20 Neon 20 Sulfur 35 Curium 248 Strontium 90 Carbon 12 Nickel 59 Questions: 1. If a nucleus has too many protons, explain what type of emission will solve the problem and why? 2. If a nucleus has too many neutrons, explain what type of emission will solve the problem and why?