pH of Biological Substances Laboratory
advertisement

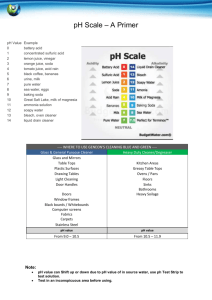
Names: ______________________________ ______________________________ Directions: Label a blank sheet of notebook paper as diagrammed on the board. Divide the paper into 8 equal squares and label them 1) lemon juice, 2) lemon juice & tums, 3) water, 4) baking soda solution, 5) ginger ale, 6) glass cleaner, 7) mustard, 8) discarded pH strips. Use a small pH strip to test each substance. Dip the pH strip half-way into your solution, pull it out and dab off any excess liquid. Place the pH strip on your scratch paper and wait about 30 seconds. Use the back of the pH tubes to estimate the substance’s pH. DO NOT touch the tube or you may blur the pH color guide (and cause your results to be inaccurate)! Lemon Juice 1) What is the approximate pH of lemon Juice ___________ 2) Why do you think that lemon juice does not burn through your throat or skin? _____________________________________________________________________ Lemon Juice & Tums 3) What is the approximate pH of the lemon juice & Tums solution 4) What would be the approximate pH of Tums (base your answers on the results you got in # 1 & # 3) ___________ ___________ 5) Tums is an “anti-acid.” What does this tell us about how Tums would react with your stomach acid when you have “heart burn?” _____________________________________________________________________ Water 6) What is the approximate pH of water? ___________ 7) Why do you think it is important that water has a relatively neutral pH? _____________________________________________________________________ Baking Soda Solution 8) What is the approximate pH of baking soda? ___________ Ginger Ale 9) What is the approximate pH of ginger ale (soda)? ___________ 10) Hypothesize what (or why) you think might cause the soda to have this pH. (Look at the ingredients on the soda bottle.) ____________________________________________________________________ Glass Cleaner 11) What is the approximate pH of glass cleaner (NH4OH)? ___________ Mustard 12) What is the approximate pH of mustard? ___________