3. Optical Properties of Glass: a) Refraction and Dispersion: A light

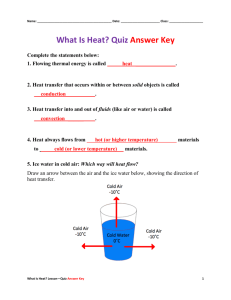
3. Optical Properties of Glass: a) Refraction and Dispersion:
A light beam is bent or refracted as it obliquely enters or leaves a piece of glass, such as a lens or a prism. The angle of incidence and angle of refraction are related by
Snell’s law
: where i and r are the angles of incidence and refraction (relative to the surface normal), n
2
is the refractive index of the glass, and n
1
is the refractive index of the surrounding medium (usually air, sometimes another glass, and sometimes another material such as water).
The refractive index n of a glass is a measure of the speed of light traveling through the glass, and is expressed as n = c / v , where c is the velocity of light in vacuum and v is the velocity in the glass. The refractive index of most glasses is greater than about 1.4. The refractive index generally depends on the wavelength of light at which it is being measured. This is because the speed of light is affected by the motions of the electrons within the glass in response to the light. These motions themselves vary depending on the wavelength of light, some electrons moving more actively at one wavelength and less at others.
Optical glasses are often described in terms of their refractive index nd at the wavelength of the helium d line, 587.6 nm.
Dispersion characterizes how the refractive index of a particular glass varies with wavelength. This is clearly an important specification for optical glasses.
In general, the index of glass decreases with increasing wavelength in an armchair shape. Refractive index increases dramatically as one approaches the ultraviolet transmission limit of the glass and dramatically decreases as one approaches the infrared region.
One measure of dispersion is the ν value, or Abbe number , which for the helium d line can be defined as ν d
= ( n d
− 1)/ ( n
F
− n
C
), where n
F is the refractive index at the hydrogen F line (486.1 nm) and n
C is the refractive index at the hydrogen C line (656.3 nm). The quantity ( n
F
− n
C
) is sometimes referred to as the principal dispersion . It can be seen that the higher the ν value, the lower the dispersion (lower the variation in index with wavelength).
Refractive indices of optical glasses range from about 1.4 to 2.4 and ν d values between about 15 and 100, although not all combinations are possible. In fact, low index in combination with high dispersion (low ν value) is difficult to achieve and high index in combination with low dispersion (high ν value) is presently essentially impossible in silicate glasses
b) Reflection:
Also important to the application of glass is the reflection of light when the light beam is incident on a surface. Reflection is not determined by the glass alone, but rather depends on the nature of the interface between that glass and the surrounding medium, such as air or vacuum. For light arriving perpendicularly to a flat, well-polished glass surface, the fraction reflected is, to a good approximation, described by the simple Fresnel formula where n g
and n a
are the refractive indices of the glass and the surrounding medium, respectively.
However, when light arrives obliquely to a glass surface, the reflectivity depends on both the angle of incidence and the state of polarization of the light, and can be calculated by using a more general form of the Fresnel equations. In all cases, the angle of incidence and angle of reflection are equal and lie in a plane containing the normal to the glass surface. This is commonly referred to a specular reflection.
However, if the glass surface is not a smooth plane, such as a ground glass surface, some of the light will be scattered diffusely, rather than reflected in a single direction. For this case, referred to as diffuse (or nonspecular) reflection, calculations of amounts and directions become much more complicated.
If a glass is not homogeneous, but rather contains a dispersion of very small particles of refractive index different from that of the glass matrix, internal reflections and backscattering of light can occur, giving the glass a translucent or even an opaque appearance. The glass will appear white or colored. To be colored, either the matrix glass, or the dispersed particles, or both, must be colored. If the glass surface is well polished, it is possible to have a portion of the light specularly reflected from the surface, and a portion diffusely reflected from below the surface.
For specular reflection of light passing perpendicularly through a sheet of window glass (refractive index about 1.5), about 4 percent of its intensity will be lost by reflection at each surface (front and back), as calculated by the simple
Fresnel formula given above, leaving only about 92 percent transmitted. c) Transmission and Absorption
Transparency (or absorption as its inverse) is one of the more important properties of glass for its application. Light travels through oxide glasses essentially unimpeded because the energy of the light is ineffective in exciting structural vibrations or causing changes in electronic energy states within the glass, either of which would result in the light being absorbed.
Oxide glasses can be considered to be wide-band-gap insulators. Silica glass, for example, has an energy band gap of about 8.5 eV, which means that the
energy difference for electrons between the highest occupied bonding orbital and the first unoccupied nonbonding orbital is about 8.5 eV. The photon energy
8.5 eV corresponds to a wavelength of about 145 nm. [For conversion, 1 eV =
1239.8/λ (λ in nm).] Only light of wavelength shorter than about 150 to 160 nm has sufficient photon energy to excite electrons from the valence band to the conduction band. Hence, the energy band gap provides the short wavelength limit to a glass’s transmission range.
At much longer wavelengths, transmission is limited because the electric field of the light can excite energy-quantized vibrations of the atoms that make up the structure of the glass. These quantized vibrations are called phonons . The absorption in this region is referred to as multiphonon absorption , since one photon of light has sufficient energy to excite two or more phonons. For pure silica, this long-wavelength, multiphonon absorption becomes significant only at wavelengths around 4 to 5 μm and greater. Hence silica is transparent throughout the entire range of the visible spectrum, plus the near infrared (IR) and much of the ultraviolet (UV) spectral regions.
If network modifiers, for example alkali or alkaline earth oxides, are added to silica, NBOs (nonbridging oxygens) are created. Recall that an NBO consists of an oxygen atom bonded to only one silicon. As such, it contains an unpaired valence electron that creates an electron energy state or level within the insulator’s band gap, thus causing absorption of light at a wavelength corresponding to the energy needed to excite this electron into the conduction band. The addition of network modifiers to silica thereby shifts the absorption edge to longer wavelengths. Transition metal ions, such as iron, which is almost universally present as an impurity in most commercial glassmaking raw materials, are capable of absorbing light through electronic transitions of inner core electrons between energy levels whose differences correspond to wavelengths in the ultraviolet, visible, or near-infrared regions. this is one of the dominant mechanisms of producing color in glass.
Absorption of light is also possible by transfer of charges (electrons) from electron shells of one ion to unfilled shells of another ion located in sufficiently close spatial proximity.
The spectral absorption bands resulting from such interionic transitions are sometimes called charge transfer bands. The participating ions may be major or minor components of the glass. Electron transfer between multivalent transition element ions, such as Fe 2+ -Fe 3+ pairs, are examples of the latter. d) Color
If the absorbance (or, inversely, the transmittance) of a glass varies with wavelength through some portion of the visible spectrum, the glass appears colored. For example, if the green and red portions of the spectrum are more strongly absorbed, then when viewed in white light, the glass will appear blue.
This is sometimes referred to as subtractive coloration. Color in glass is usually
caused by the intentional, or sometimes unintentional, inclusion of absorbing ions, which are generally transition metal ions, in the composition. Since the absorbed wavelength corresponds to the electron transitions allowed, the resulting color produced is a function of the oxidation state of the transition metal ion. The bluish-green color noted in a piece of window glass viewed through its edge is primarily due to an absorption at 1.1-μm wavelength by Fe 2+ iron present as an impurity in most commercial glass-making raw materials. By melting the glass in a more oxidizing atmosphere, some of the Fe 2+ converts to
Fe 3+ . The absorption due to a charge transfer between Fe 2+ and Fe 3+ is in the ultraviolet, tailing into the visible region. This results in a straw-yellow color.
By a judicious control of the Fe 3+ /Fe 2+ ratio, the straw-yellow color due to
Fe 3+ ⇔ Fe 2+ and the bluish-green color due to Fe 2+ complement each other to bring about decolorization of glass.
Dispersing colloidalsized particles, or pigments, within the glass can also produce color. These pigments can be oxide, semiconductor or metal, and can be mixed into the glass, as with some types of vitreous glazes, or chemically precipitated after having been put into solution at high temperatures in the melt.
The red color obtained as a result of the presence of approximately 200-Å gold particles in glass.
4. Electrical Properties of Glass: a) Electrical Conduction
Ionic motion is the dominant diffusion phenomenon in silicate glasses. Of these, conduction by alkali ions makes up 99 percent or better of the electrical transport in common silicate glasses. Hence, ions are the most mobile charge carriers.
Since ions are less mobile than electrons, the silicate glasses are basically electrical insulators relative to the metals, where electrons are the dominant charge carriers. There is some electronic conduction in silicate glasses, particularly in those that have a significant amount of transition metals, such as
Fe, Mn, and V, displaying multivalency in glass. Electronic conduction is predominant in elemental glasses, such as silicon, germanium, and the chalcogenides, where such conduction is, in fact, responsible for the semiconductor properties, and, perhaps, for much of the modern electronic industry.
Glasses containing fluoride ions display anionic conduction based on the transport of F
−
. Some silver- and copper-containing glasses display fast ion conduction based on transport of charge by Ag + and Cu + ions.
The basic equation describing electrical conduction is Ohm’s law, according to which the resistance Re (ohms) is given by
V is the electrical potential (volts) applied across the conductor and I is the current (in amperes) that flows. The resistance for a conductor having a crosssectional area A and length L is given by where ρ is called the resistivity
. Appropriate units for ρ are Ω·cm (in cgs) or
Ω·m (in SI). Conductivity σ is the reciprocal of resistivity; the units are often written as mho/cm or S/m (siemens per meter).
Application of DC Potential across Glass
When a DC potential is applied across a thin disk of a silicate glass, the mobile alkali cations (e.g., Na + ) migrate from the anode region to the cathode region.
The two nonbridging oxygen anions, to which the two Na recombine to form one bridging oxygen and release one O
+
2−
were attached,
, which moves toward the anode. Glass is thus gradually electrolyzed. The electrode reactions are:
Thus, metallic sodium is deposited on the cathode plate and oxygen is outgassed from the anode region. The depletion of ions near each electrode builds a polarization field , which causes a rapid decrease of current. In time, the
current stops flowing. Oxygen outgassing and the motion of alkali ions, both under the influence of electric field, are a serious problem when glass is bombarded by charged particles, such as electrons in a television picture tube or in instruments such as an electron microscope or an electron probe analyzer. b) Dielectric Properties
When a dc voltage V is applied to a large parallel plate capacitor having a narrow vacuum gap d between the plates, then the plates are charged with and
− q charges per unit area immediately. The electric displacement flux D
0
+ q
that develops may be given by:
D
0
q
The electric field strength E is defined by
D
0
0
E
and
E
V
/
d where ε
0
is called the absolute permittivity (dielectric constant) of the free space, and has a value 8.854 × 10
−12
F/m. The capacitance C
0
per unit area between the parallel plates is given by
C
0
= ε
0
/
d
If we replace vacuum by a dielectric such as glass, the new capacitance C and electric displacement are given by
C
= ε
s
C
0
and
D
= ε
s
D
0 where ε s
is called the static relative permittivity (or the relative dielectric constant). Thus,
D
= ε
s
ε
0
E
= ε
E where ε is called the electric permittivity of the medium.
In an alternating field, we may write
D
* = ε*ε
0
E
*
where the asterisk is used to represent complex numbers. Thus, the complex dielectric constant has the form
ε * = ε ′−
j
ε ″
where j = (−1) 1/2 . The real part ε ′ is the dielectric constant, and the imaginary part ε″ is the dielectric loss factor. The ratio
ε ″/ε ′ = tan δ
is termed the loss angle , where δ is the phase difference angle between the electric field E * and the electric displacement flux D *. Dielectric properties are measured as a function of the applied frequency by ac bridges or other electronic equivalents.
All dielectrics suffer from breakdown when the applied voltage exceeds the dielectric strength. The breakdown usually results in a changeover from an insulating to a conducting behavior, often because of a large dielectric loss factor. Dielectric constants are important for many applications. Large
capacitors require high dielectric constants. On the other hand, in integrated circuit applications, the substrates are required to have low dielectric constants to allow a high interconnect signal speed.
The dielectric constant of glasses is generally 4 to 11 at 1 MHz and 20°C.
Typical values for soda-lime-silicates are 7 to 9. Some internally nucleated glass-ceramics in the B
2
O
3
-P
2
O
5
-SiO
2
system have values as low as 3.8 to 4.5, suggesting potential applications as microelectronic substrates, replacing alumina for which the dielectric constant is 9. The loss angle, tan δ, is strongly related to the conductivity σ. Values are generally 10 −3
and increase with the amount of alkali ions, again mitigated by the mixed-alkali effect. Presence of heavy metal ions, such as Pb, causes a decrease in tan δ.