Absorbance and the Beer
advertisement
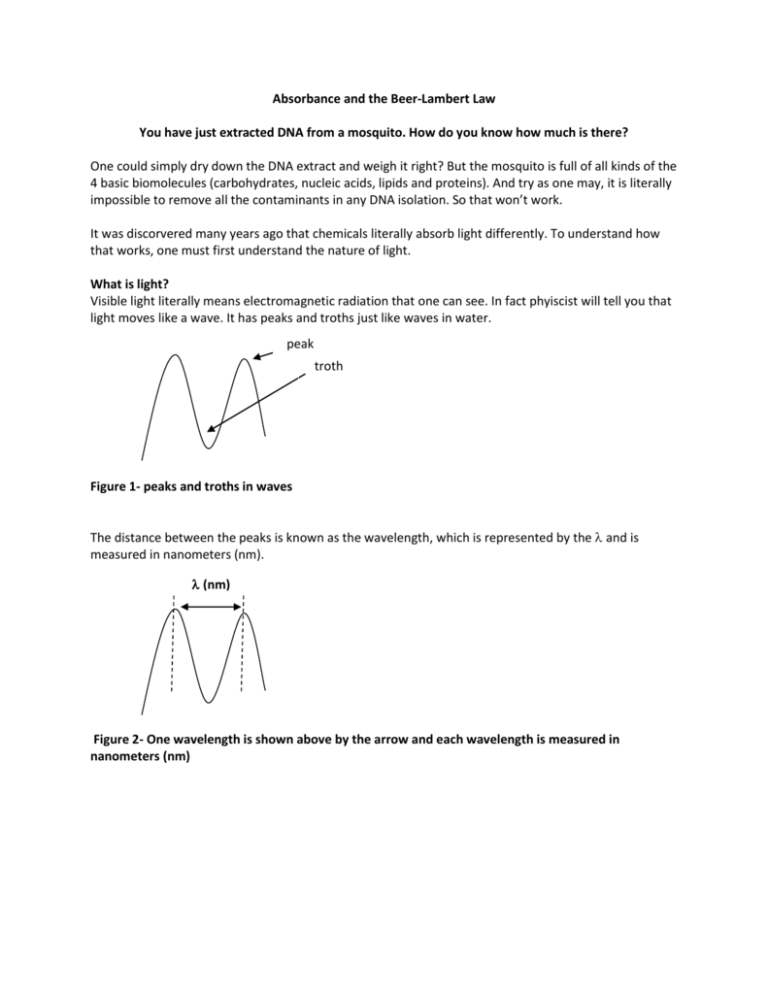
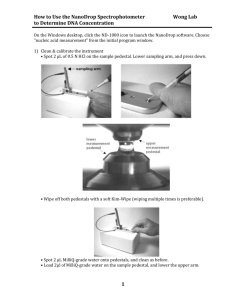
Absorbance and the Beer-Lambert Law You have just extracted DNA from a mosquito. How do you know how much is there? One could simply dry down the DNA extract and weigh it right? But the mosquito is full of all kinds of the 4 basic biomolecules (carbohydrates, nucleic acids, lipids and proteins). And try as one may, it is literally impossible to remove all the contaminants in any DNA isolation. So that won’t work. It was discorvered many years ago that chemicals literally absorb light differently. To understand how that works, one must first understand the nature of light. What is light? Visible light literally means electromagnetic radiation that one can see. In fact phyiscist will tell you that light moves like a wave. It has peaks and troths just like waves in water. peak troth Figure 1- peaks and troths in waves The distance between the peaks is known as the wavelength, which is represented by the and is measured in nanometers (nm). (nm) Figure 2- One wavelength is shown above by the arrow and each wavelength is measured in nanometers (nm) Light in the visible spectrum is a composite of many different wavelengths of light, which a prism will show you as different colors as shown below (violet to red). There are other types of electromagnetic radiation as well, like ultraviolet light (uv). The shorter the wavelength the greater the energy the electromagnetic radiation has, so gamma rays ( rays in Figure 3, note the arrow) is really high energy radiation. This is the one that could fry someone in a nuclear reactor. The red light in the visible light spectrum has much lower energy and much longer wavelengths. Figure 3- Spectra of Electromagnetic Radiation (http://en.wikipedia.org/wiki/File:EM_spectrum.svg) It turns out that molecules can absorb light at different wavelengths which makes it possible to determine if one type of molecule is in a large mixture of molecules. For example, proteins tend to absorb light with a wavelength of 280 nm (or we would say “absorb light at 280”). On the other hand nucleic acids absorb light at 260nm. You will notice that the visible spectrum above ranges from about 380 nm to roughly 700 nm. So 260 nm and 280 nm are ultraviolet light (uv). After all ultra means “more than” and ultraviolet light has shorter wavelengths and thus more energy than violet light. If only one could measure absorbance! Well one can measure the absorbance of a solution in a spectrophotometer. In a nutshell, a spec (short for spectrophotometer) can filter light from a bulb to give the required wavelength of light. It shines the light so it passes through the sample which is usually in a cuvette. The molecules in the sample will absorb some of the light. The rest will pass through and hit a detector which in turn will measure the amount of light hitting it and give you a unitless number from 0.0 to 2.0 (see Figure 4). Figure 4- Light in a Spectrophotometer (http://www.bing.com/images/search?q=spectrophotometer+and+picture&view=detail&id=555BCD6EEFEB61293BED8E4268F6AD438AA34 78E&first=1) Absorbance Spectra If you graph the absorbance of a protein sample stimulated with different wavelengths of light one will see the following type of graph. A 240 250 260 270 280 290 300 310 320 Wavelength (nm) Absorbance Maximum at 280nm B 240 250 260 270 280 290 300 310 320 Wavelength (nm) Figure 5- A Typical Absorption Spectra of Protein and DNA- Note the maximum absorbance at 280 m (Panel A) which indicates protein in the sample. In Panel B the peaks at 260 nm and 280 nm indicate nucleic acids and proteins in the sample. Using Absorbance to Measure Concentration of Chemicals Many years ago two people Beer and Lambert determined that the concentration of a chemical can be measured with absorbance. In fact they were directly proportional, meaning that if concentration says doubles, the absorbance will also double. They described this relationship in the Beer Lambert Equation. A = ebc absorbance = molar extinction coefficient* x length of light path** x molar concentration extinction coefficient units are L/mole cm length of light path measured in cm molar concentration (moles/L) * n.b. The molar extinction coefficient indicates the ability of the molecule to absorb light at a certain wavelength. One looks this number up. **n.b. The length of the light path has a defined and measurable length measured in cm What you need to pay attention to is that when A A A increases c will increase the same amount. If doubles so does C. If increases 1.25 times, so does C, etc. Bottom line, one can use a spec to measure the absorbance of a sample at 280 nm and 260 nm to estimate how much protein and nucleic acid is in a sample. To do this for your mosquito DNA samples you will use a machine called a nanodrop. This very expensive and delicate technology is a spec that can measure nucleic acid concentrations in very small volumes. In fact only 0.5 to 1 ml is needed! That means you only need 0.0000005 to 0.000001 L! As you will find-out your samples are precious. Sometimes one can only get a few ml of samples and they may even be one of a kind! So they can be precious! Arm A B Figure 6- Pictures of a nanodrop- Panel A shows the arm (note the arrow). Panel B shows a sample being pipette on the pedestal. (http://www.nanodrop.com/Productnd2000coverview.aspx) Procedure to use the nanodrop 1. Blank the instrument. Even the liquid your sample is in (the solvent) absorbs light. One must tell the machine not to detect those molecules If the DNA is dissolved in ultrapure water, then use that to blank the nanodrop. Gently lift the arm, place 1 l of solvent on the pedestal with a micropipetor and very gently lower the arm. Click on the blank button on the computer. Click on the run button on the computer to ensure the result will read and absorbance of zero. 2. Read the sample Gently lift the arm, place 1 l of the sample on the pedestal and very gently lower the arm Click on the run button on the computer 3) The computer will show a value for concentration that is in ng/l. 4) It will also show a 260/280 ratio, which is the ratio of absorbances at those wavelengths. Clean DNA will have a 260/280 ratio from 1.5 to 1.8. 5) The absorbance spectra will probably show at least 3 peaks. The one around 230 is for contaminants from the DNA isolation or carbohydrates in the sample. Roughly speaking, the peak at 260 nm (DNA) should be as large or at least ½ the size of the peak at 230 nm for the next experiments to work. It is important to realize that the Beer Lambert Law is used by the computer to calculate the amount of DNA in the sample. The computer program has already been set with the path length for the nanodrop and molar extinction coefficient for DNA. Activity Your instructor will introduce you to using the p20 micropipetor. Practice taking a nanodrop reading for the 3 DNA samples provided. Explain whether or not you think the samples will be good ones for our future work. Data Table Sample # 1 2 3 A 260 A 280 260/280 230/260 Explain why you think it will or will not work?