Results and Discussion - World Journal of Engineering
advertisement

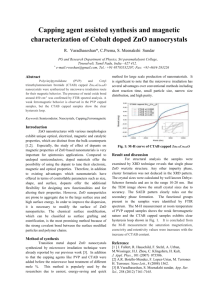
World Journal Of Engineering Increased thermal stability of multi-walled carbon nanotube structure by attaching CTAB capped zinc oxide nanoparticles Manish Kumara, Mandeep Singhb, Dushyant Kushavaha, M.L Singlaa a Material Research Division, Central Scientific Instruments Organization (CSIO), Chandigarh (India) b Department of Chemical Engineering, Institute of Chemical Technology, Prague (Czech Republic). Email:mandeep.singh@vscht.cz Introduction Results and Discussion In the XRD patterns were recorded by means of a Rigaku D max III C diffractometer in fig 1(a) shows all the major peaks of the ZnO nanoparticles at 31.7°, 34.34°, 36.18°, 47.47°, 56.53°, 62.79° and 67.89° were assigned to (100), (002), (101), (102), (110), (103) and (112) planes of hexagonal wurtzite structured of ZnO (JCPDS Card File No. 36-1451) accept one peak at 26.56° corresponding to MWCNT at (002) plane indicating that the composite is a contribution of CNT and ZnO nanoparticles and fig 1(b inset) is the TEM image (using JEOL – 1200EX) of the composite clearly shows the attachment of CTAB capped ZnO nanoparticle onto MWCNT scaffolds. Thus, TEM and XRD structural study reveals the formation of the composite powder. The properties of multi-wall carbon nanotubes (MWCNT’s) such as high mechanical strength or high thermal and electrical conductivity can be further enhanced by the addition of both organic and inorganic substances to form composite structures. In particular, CNT’s loaded with various types of nanoparticles like Au [1], Ag [2], metal oxides TiO2 [3], and ZnO [4] by using approaches including electrostatic coordination [5], thermal decomposition [1], seed-mediated growth [6] etc. In the present work, we report a new and relatively facile approach to prepare MWCNT’s composites using CTAB coated ZnO nanoparticles. Here, CTAB (cationic surfactant) capped ZnO nanoparticles attached the nanotube surface due to hydrophobic and electrostatic interactions. Further, a detailed microscopic and thermal study of the prepared composite material is presented and the key structural and functional aspects of the composite are discussed. Material and Methods Zinc acetate dihydrate, Sodium hydroxide (NaOH), Cetyl trimethylammonium bromide (CTAB), Nitric acid (HNO3), Hydrochloric acid (HCl), and Isopropanol were used as received. MWCNT’s were purchased from Nanostrurtured & Amporphous materials Inc. (US). For the synthesis of composite, as-received MWNTs were first treated by refluxing in HNO3 (40%) at 110 °C for 2 hours to improve their dispersibility in aqueous solution by forming oxygen containing functional groups on their side walls. Acid treated MWNTs (10 mg) were added into 40 mL distilled H2O and ultrasonicated for 15 min. HCl (38%, 0.7 ml) was added during the ultrasonication (solution A). CTAB coated ZnO nanoparticles have been synthesized according to our previously reported method [7]. Nanoparticles were then added to acid-treated MWNT’s from solution A under vigorous stirring and the stirring continued for 2 h. The mixture was then transferred to a 40 mL Teflon-lined stainless steel autoclave and heated at 150 °C for 20 hours. The final product was washed repeatedly with distilled water and absolute alcohol and dried at 60 °C in vacuum. Fig.1 (a-b) XRD pattern and TEM image of the composite The TGA-DSC measurements were carried out on dry powder samples heated at a ramping rate of 5 °C/min in the presence of an inert atmosphere using SDT-Q600 TA instrument to study their thermal structural stability. Figure 2(a) shows the TGA-DSC curve of the as received MWCNT’s, where 65% reduction in weight from 450 °C to 700 °C (DSC maximum 600°C), most likely due to the decomposition of the CNT’s. Figure 2(b) of CTAB coated ZnO nanoparticles shows first decrease of 2.5 % below 150 °C due to the release of residual solvent and adsorbed water, the second decrease of 5.7 % till 500 °C is due to desorption and decomposition of the surfactant and further decrease is due to the de-hydroxylation of the surface and the removal of the residual surfactant 613 World Journal Of Engineering Conclusions (DSC maximum 460°C). Figure 2(c) of their composite the initial weight loss of 5.5 % up to 400 °C is due to the release of the residual solvents and adsorbed water, the desorption and decomposition of the surfactant and also the decomposition of the – COOH group of the acid treated MWCNT’s. Further decrease of 19.8 % up to 700 °C attributed to the dehydroxylation of the ZnO nanoparticle surface plus the removal of the any residual surfactant and also the decomposition of the nanotubes (DSC maximum 580°C). The thermal stability of the MWCNT is remarkably improved upto 40% by nanoparticles incorporation. CTAB capped ZnO nanoparticles with MWCNT composite powder was synthesized in facile manner. XRD and TEM analysis reveals the formation of the composite powder. The thermal stability of the MWCNT structure increased by 40% after the incorporation of CTAB capped ZnO nanoparticles. As the basic thermal properties of ZnO nanoparticles remain preserved in the composite, the present composite is a suitable candidate for low- to medium temperature applications while retaining the thermal properties of the individual components – in particular the carbon nanotube network in the composite material and for biomedical application-oriented studies as Protein resistant CNT–polymer composites References [1] Zhang, M., Su, L. and Mao, L. Surfactant functionalization of carbon nanotubes (CNTs) for layer by-layer assembling of CNT multi-layer films and fabrication of gold nanoparticle/CNT nanohybrid. Carbon 44 (2006) 276-283. [2] Liu, Y., Tang, J., Chen, X.Q., W. Chen, Pang, G.K.H. and Xin, J.H. A wet-chemical route for the decoration of CNTs with silver nanoparticles. Carbon 44 (2006) 381-383. [3] Jitianu, A., Cacciaguerra, T., Benoit, R., Delpeux, S., Beguin, F. and Bonnamy, S. Synthesis and characterization of carbon nanotubes-TiO2 nanocomposites. Carbon 42 (2004) 1147-1151. [4] Chen, C.S., Chen, X.H., Yi, B., Liu, T.G., Li, W.H., Xu, L.S., Yang, Z., H. Zhang, H. and Wang, Y.G. Zinc oxide nanoparticle decorated multi-walled carbon nanotubes and their optical properties. Acta Materialia 54 (2006) 5401-5407. [5] Ravindran, S. and Ozkan, C.S. Self-assembly of ZnO nanoparticles to electrostatic coordination sites of functionalized carbon nanotubes. Nanotechnology 16 (2005) 1130-1136. [6] Li, C., Jin, Z., Chu and H.,Li, Y. Seed-mediated growth of ZnO nanorods on multiwalled carbon nanotubes. J. Nanosci. Nanotechnol. 8 (2008) 44414446. [7] Singla, M.L., Shafeeq, M. and Kumar, M. Optical characterization of ZnO nanoparticles capped with various surfactants. J. Luminescence 129 (2009) 434438. Fig.2 (a-c) TGA-DSC of (a) MWCNT, (b) CTAB/ZnO nanoparticles and (c) their composite 614