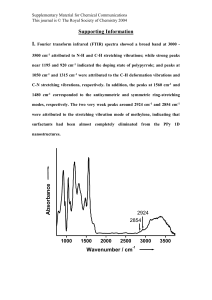
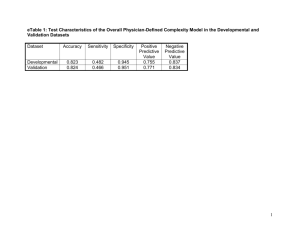
SPECTROSCOPIC INVESTIGATION OF THE ELECTRON
advertisement

STUDIA UNIVERSITATIS BABEŞ-BOLYAI, PHYSICA, SPECIAL ISSUE, 2001 SPECTROSCOPIC INVESTIGATION OF THE ELECTRON DELOCALIZATION AND MOLECULAR CONFORMATIONAL CHANGES IN POLYPYRROLE INDUCED BY DIFFERENT DOPING IONS R. TURCU*, R. GRECU**, M. BRIE**, I. PETER*, A. BOT*, W. GRAUPNER*** * National Institute of R & D for Isotopic and Molecular Technologies, P.O. Box 700, 3400 Cluj-Napoca, Romania ** Institute of Chemistry "Raluca Ripan", 3400 Cluj-Napoca, Romania *** Institut fur Festkorperphysik, Technische University Graz, A-8010 Graz, Austria ABSTRACT. We report a comparative study of the optical properties of polypyrrole (PPY) synthesized by two methods: electrochemical and chemical oxidation of the monomer (pyrrole) in the presence of different doping ions (p-toluensulfonate, dodecylsulfate, dodecylbenzenesulfonate, perchlorate, tetrafluoroborate). By using FTIR and UV-Vis spectroscopy we investigated the changes of the molecular and electronic structure of polypyrrole induced by the preparation conditions. Our main goal was to find out the relevant synthesis parameters, which have a strong influence on the electron delocalization length and the molecular architecture of polypyrrole. 1. Introduction Conducting polymers are new materials that combine the mechanical and chemical properties of isolator polymers with the electrical and optical properties of inorganic semiconductors and metals. Polypyrrole is one of the most used conducting polymers for applications due to the good stability of his properties and because it can be easy synthesised as a homopolymer and also as a composite. The success of PPY applications depends on the improvement of the properties and the processability of this material. Therefore one of the main research goal is the correlation between the synthesis parameters and the molecular architecture of PPY in order to obtain the required properties for a specific application [1]. In this paper we report a comparative study of the optical properties of polypyrrole (PPY) synthesized by two methods: electrochemical and chemical oxidation of the monomer (pyrrole) in the presence of different doping ions (p-toluensulfonate, dodecylbenzenesulfonate, perchlorate, tetrafluoroborate). By using FTIR, UV-Vis spectroscopy we investigated the changes of the molecular and electronic structure of polypyrrole induced by the preparation conditions. Our main goal was to find out the relevant synthesis parameters, which have a strong influence on the electron delocalization length and the molecular architecture of polypyrrole. SPECTROSCOPIC INVESTIGATION OF THE ELECTRON DELOCALIZATION … 2. Experimental 2.1 Samples preparation a) Electrochemical synthesis of polypyrrole The electrochemical synthesis of polypyrrole was carried out with different electrolyte types: p-toluenesulfonic acid (HTSO), sodium toluenesulfonate (NaTSO), lithium perchlorate (LiClO4) and tetrabutyl-ammonium-tetrafluoroborat (Bu4 NBF4), sodium dodecylbenzenesulfonate (DBSNa). The electrolyte concentration, ce was varied in the range 0.01-0.15 M. The polymerization was carried out in galvanostatic conditions at room temperature using different current densities: 0.25 j 1.25 mA/cm2. Polypyrrole films with thickness h in the range 10-25 m were obtained. b) Chemical synthesis of polypyrrole The monomer pyrrole and the dopant DBSNa were mixed vigorously with distilled water. The water solution of the oxidant ammonium persulfate (APS) was added by dropping to the pyrrole-DBSNa solution. The polymerization was performed by using the oxidant/monomer (O/M) molar ratio 0.2 and dopant/monomer (D/M) molar ratio 0.5 or 0.8. The mixture was stirred out at 15C for 24 hours and adding excess methanol to the reaction flask terminated the polymerisation of pyrrole. PPY was formed as a black powder, which was filtered and washed successively with distilled water, methanol and acetone, followed by filtering and drying in a vacuum oven at 30C for 14 hours. PPY chemically synthesised using large-size protonic acid dopants such as DBS is soluble in organic solvents like m-cresol and chloroform. The polypyrrole fine black powder (100 mg) was dissolved in 2 ml of chloroform or m-cresol. As was previously reported by J.Y. Lee et al., PPY(DBS) has a lower solubility in chloroform than in m-cresol [2]. To improve the solubility of PPY powder DBSNa (100 mg) was added to the solution. The solutions were stirred for 18 hours and then filtered through a Millipore filter paper. Thin films of polypyrrole were cast from these solutions on glass or ITO (indium tin oxide) covered polycarbonate supports for UV-Vis transmission measurements. 2.2 Optical measurements Specular reflectance measurements in the range 200 - 6000 cm-1 were performed on free-standing PPY films by FTIR spectroscopy on a BOMEM MB-102 spectrophotometer. The absolute value of sample reflectivity was determined relative to the reflectivity of an aluminium mirror. The optical constants for PPY films were calculated from the reflectance spectra with Kramers-Kronig (K-K) technique [3]. Infrared transmission spectra in the range 400-4000 cm-1 were recorded by a spectrophotometer JASCO FTIR-610, on pressed pellet prepared from the chemically prepared PPY(DBS) powder embedded in KBr or on thin film cast on KBr crystal from the filtered solution of PPY in chloroform. UV-Vis transmission spectra of the as-prepared thin polymer films on glass support were recorded by a Lambda 9 spectrophotometer. 217 R. TURCU, R. GRECU, M. BRIE, I. PETER, A. BOT, W. GRAUPNER 3. Results and discussion 3.1 Reflectance spectra of the electrochemically prepared PPY The reflection spectra of polypyrrole samples, shown in Fig.1 are sensitive to the nature of the electrolyte and to the d.c. conductivity values. The higher reflectivity was obtained for TSO doped PPY samples. Significantly lower reflectivity was obtained for sample 4, with the lower dc. These results demonstrate that the surface morphology of the sample has a significant contribution to the reflectance. We consider that the roughness of the film surface depends on the nature and concentration of the electrolyte. The surface roughness is lower for TSO- doped film as compared with ClO4- doped one and decreases with the decrease of the electrolyte concentration. 70 (1) (2) (3) (4) Reflection (%) 60 50 = 14 S/cm = 77 S/cm = 22 S/cm = 11 S/cm 40 30 (1) 20 (2) (3) 10 (4) 0 0 1000 2000 3000 4000 5000 6000 -1 Wavenumber [ cm ] Fig.1 Reflection spectra of PPY films electrochemically prepared with different electrolytes: 1- NaTSO ; 2-HTSO ; 3- LiClO4; 4-NaDBS The increase of the reflectance at lower frequencies indicates a metal-like behavior. But to find out if the electrons are indeed delocalized in our samples we have to determine the behavior of the dielectric function in the investigated spectral range. The real and imaginary parts of the refractive index, n() and k() were calculated from the reflection spectra with the Kramers-Kronig technique [3], where first the phase angle is determined using the following relationship: 0 0 d log R 1 log d 2 0 0 d Then we can determine n and k : 218 (1) SPECTROSCOPIC INVESTIGATION OF THE ELECTRON DELOCALIZATION … n 1 R 1 R 2 R cos k 2 R sin 1 R 2 R cos (2) For this purpose we have to extrapolate the experimental data to zero and infinite energies (wavenumbers). For the low energies a Hagens-Rubens function was used [3, 4], as the reflectance increases to low energies for all films investigated. To high energies the data were extrapolated by a decay function (-s). The spectral dependencies of n and k for TSO- doped polypyrrole sample 2 are shown in Fig.2. Similar behaviour for n and k was obtained for the other polypyrrole samples investigated [4]. Several peaks located at 850, 1018, 1150, 1300, 1450 and 1530 cm-1, which correspond to the characteristic absorption peaks of the pyrrole ring, can be observed in the spectral dependence of k (Fig.2). The values of k() increase with the increasing of the d.c. conductivity values of the samples. The band located at 1530 cm-1 is attributed to the collective vibration of C=C/C-C inter and intra-rings. The characteristic frequency of this band is related to the conjugation length on the chains: the lower is the frequency, the higher is the conjugation length. We can estimate a conjugation length of 9-10 rings for our electrochemically prepared PPY [1,4]. 6 Refractive index 5 4 3 n 2 k 1 400 600 800 1000 1200 1400 1600 1800 2000 -1 Wavenumber (cm ) Fig.2 Real part (n) and imaginary part (k) of the refractive index for PPY (sample 2 from Fig.1) The complex dielectric function, is defined by: 1 i2 1 i 0 (3) The real and imaginary part of the dielectric function were calculated from n and k values, using the relations: 219 R. TURCU, R. GRECU, M. BRIE, I. PETER, A. BOT, W. GRAUPNER 1 n 2 k 2 ; 2 2 nk 0 (4) From Fig.3 one can observe that the real part of the dielectric function 1 is positive in all the spectral range. This behavior indicates that most charge carriers are localized in our PPY samples. 25 200 20 150 100 15 50 10 0 0 1000 2000 3000 4000 5000 6000 -1 Wavenumber (cm ) 5 0 0 1000 2000 3000 4000 5000 6000 -1 Wavenumber (cm ) Fig.3 Real and imaginary parts of the complex dielectric function for PPY (sample 2 from Fig.1) 3.2 Optical properties of the chemically prepared PPY UV-vis absorption spectra The absorption spectra of PPY(DBS) thin films cast from chloroform and mcresol filtered solutions (100 mg PPY1 (DBS) powder in 2 ml of solvent) are presented in the Fig 4. It is well know that two characteristic bipolaronic absorption bands located at ~ 1 eV and ~ 2.6 eV appears in the optical spectra of doped PPY [6]. A shift of these bipolaronic bands could appear as a function of the doping level. From the Fig. 4 one can see that the bipolaronic absorption band located at 2.6eV for PPY(DBS) obtained from chloroform solution is shifted to higher energies for the sample obtained from m-cresol solution. The shift of the absorption band could be attributed to the polymer-solvent interaction. The nature of the solvent molecule influences the polymer chains conformation and the length of the conjugated segments leading to a difference in the energy gaps. We consider that PPY(DBS) cast from mcresol solution contain shorter conjugated segments as compared with PPY(DBS) cast from chloroform solution [7]. 220 SPECTROSCOPIC INVESTIGATION OF THE ELECTRON DELOCALIZATION … 1.1 Normalized absorbance 1.0 0.9 a 0.8 0.7 b 0.6 0.5 1.4 1.6 1.8 2.0 2.2 2.4 2.6 2.8 3.0 3.2 3.4 E(eV) Fig.4 Normalized absorbance of PPY(DBS) films cast from: a- chloroform solution; b- m-cresol solution IR spectra In Fig. 5 we can compare the infrared spectra for the as-synthesised PPY(DBS), spectrum A and for the soluble fraction, spectrum B obtained by the filtration of PPY(DBS) chloroform solution. Both spectra show an increase of the absorbance in the range 1700-4000 cm-1 , which is attributed to the tail of the electronic transition located in the near infrared. The absorbance increases more slowly in this region for the soluble fraction PPY(DBS) as compared to the as-synthesised sample. This behaviour could be due to a lower oxidation degree or to a decrease of the electrons delocalization along the chains of the soluble fraction. The lower contribution of the electronic transitions involving charge carriers make a clear evidence possible in the spectrum B of the absorption bands located near 2900 cm-1 attributed to the vibrations of C-H groups in the dopant DBS. Moreover, the stretching vibrations of the N-H group in PPY can be observed as a shoulder near 3300 cm-1 in the spectrum B. The significant differences between the spectra A and B appear for the bands located around 900 cm-1, 1200 cm-1 and 1550 cm-1, see the inset in the Fig. 5. These absorption bands characteristic for the oxidized PPY have been associated by Zerbi with the effective conjugation coordination and show sensitivity to the oxidation level and to the conjugation length of the chain [9]. The band located at 1550 cm-1 in the spectrum A is attributed to C=C/C-C stretching vibrations of the PPY chain [9]. The shift of this band to a higher frequency, 1569 cm-1 in the spectrum B indicate that soluble PPY(DBS) contain mainly shorter conjugated chains as compared with the as-prepared PPY . The absorption band located at 1198 cm-1 in the spectrum A are ascribed to ring breathing vibrations and its shift to higher frequency in the spectrum B support the idea of the lower oxidation level for the soluble fraction as compared to the assynthesised PPY. 221 R. TURCU, R. GRECU, M. BRIE, I. PETER, A. BOT, W. GRAUPNER From the inset in the Fig. 5 one can observe that the intense band located at 914 cm-1 in the spectrum A has a significant shift to higher frequency (by 26 cm-1) in the spectrum B. This band could be attributed to a PPY ring vibration mode in the oxidation state. This assignment is based on the fact that ring vibrations are much more sensitive to the oxidation level and to the conjugation length of the chains as compared to C-H vibrations [9]. 2.2 1.8 1.6 Absorbance 1.2 2.0 A 1.0 0.8 B 0.6 0.4 0.2 900 Absorbance 1000 1100 1200 1300 1400 1500 1600 -1 1.4 wavenumber (cm ) A 1.2 1.0 0.8 0.6 B 0.4 0.2 500 1000 1500 2000 2500 3000 3500 4000 -1 wavenumber (cm ) Fig.5 IR spectra of PPY(DBS): A-as-synthesised sample; B-soluble fraction in chloroform The comparison of the IR spectra for the chemically prepared soluble PPY(DBS) and the electrochemically prepared PPY doped with different ions (TSO, ClO4, BF4, DBS) shows that the former contains mainly shorter conjugation lengths and has a lower degree of oxidation. R E FER EN CE S 1. 2. 3. 4. 5. 6. 7. 8. 222 J. Rodriquez, H. J. Grande, T.F. Otero, "Polypyrroles: from Basic Research to Technological Applications", in: Handbook of Organic Conductive Molecules and Polymers, ed. H.S. Nalwa, John Wiley & Sons, 1997, Vol.2, p.415 J.Y. Lee, D.Y. Kim, C.Y. Kim, Synth. Met. 74, (1995), 103 G. Leising, in "Organic Materials for Photonics", ed.G.Zerbi, Elsevier, Amsterdam 1993.p.173 R.Turcu, M.Brie, G.Leising, V.Tosa, A.Mihut, A.Niko, A.Bot, Appl. Phys. A67, (1998), 283 R.S. Kohlman, T.Ishiguro, H.Kaneko, A.J. Epstein, Synth. Met. 69, (1995), 325 O. Chauvet, S. Paschen, L. Forro, L. Zuppiroli, Synth. Met. (1994) 63, 115 R.Turcu, W.Graupner, C.Filip, A.Bot, M.Brie, R.Grecu, Adv.Mater. Opt. Electron.(1999), 9, 157 G.Zerbi, M. Gussoni, C. Castiglioni, Conjugated Polymers; Ed. J.L.Bredas, R.Silbey, Kluwer Academic Publishers, 1991; 435-507