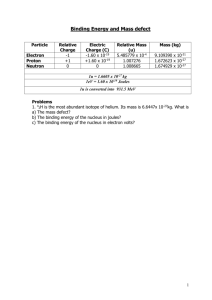
1 - TheWorldaccordingtoHughes
advertisement
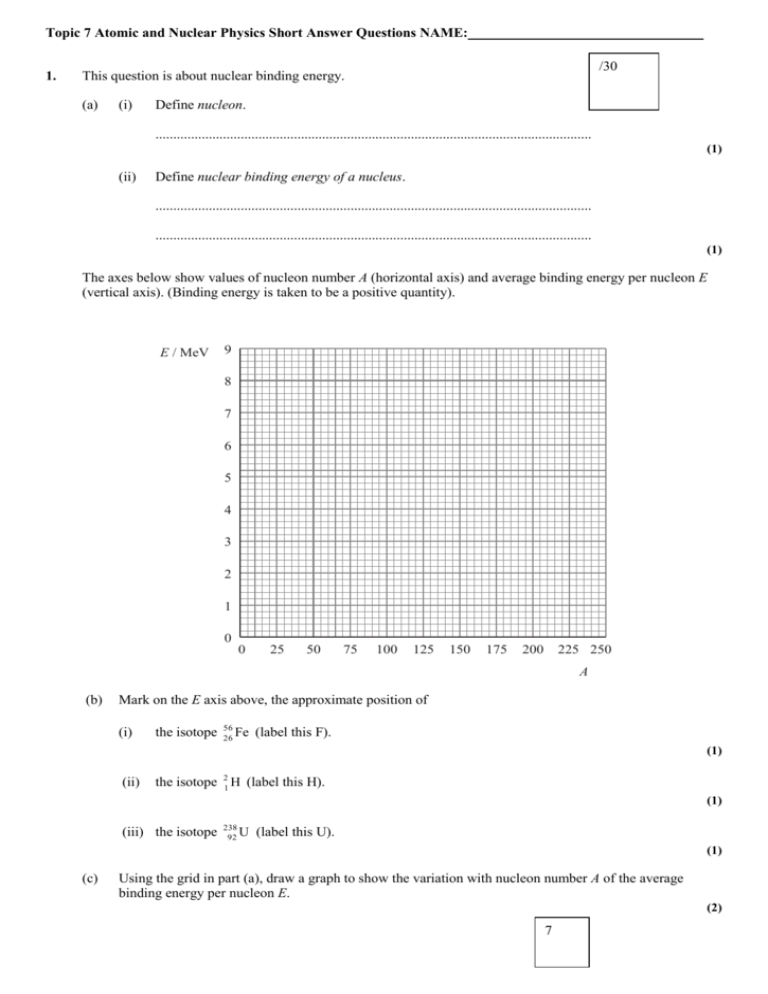
Topic 7 Atomic and Nuclear Physics Short Answer Questions NAME: 1. /30 This question is about nuclear binding energy. (a) (i) Define nucleon. ........................................................................................................................... (1) (ii) Define nuclear binding energy of a nucleus. ........................................................................................................................... ........................................................................................................................... (1) The axes below show values of nucleon number A (horizontal axis) and average binding energy per nucleon E (vertical axis). (Binding energy is taken to be a positive quantity). E / MeV 9 8 7 6 5 4 3 2 1 0 0 25 50 75 100 125 150 175 200 225 250 A (b) Mark on the E axis above, the approximate position of (i) the isotope 56 Fe 26 (label this F). (1) (ii) the isotope 21 H (label this H). (1) (iii) the isotope 238 U 92 (label this U). (1) (c) Using the grid in part (a), draw a graph to show the variation with nucleon number A of the average binding energy per nucleon E. (2) 7 (d) Use the following data to deduce that the binding energy per nucleon of the isotope 23 He is 2.2 MeV. nuclear mass of 23 He = 3.01603 u mass of proton = 1.00728 u mass of neutron = 1.00867 u ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) In the nuclear reaction 21 H 21 H 23 He 01 n energy is released. (e) (i) State the name of this type of reaction. (1) ........................................................................................................................... (ii) Use your graph in (c) to explain why energy is released in this reaction. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... 2. (2) When the electron was first discovered it led to the idea that an atom consists of a lump of positive charge in which the electrons are embedded. In 1912 Geiger and Marsden carried out an experiment to test the validity of this idea. The results of their experiment in fact suggested that the atom is mostly empty space with an electrically charged nucleus of relatively large mass occupying only a small amount of space. (This is the socalled nuclear model of the atom). Their experiment involved “firing” alpha particles at a thin sheet of gold foil. (a) State the nature of an alpha particle. ..................................................................................................................................... (1) The diagram below shows a small part of the gold foil with two alpha particles A and B approaching the foil. A B gold foil 7 2 (b) (i) Some alpha particle trajectories lead to the idea that most of the atom is empty space. On the diagram, draw such a trajectory for the alpha particle A. (1) (ii) Some other alpha particle trajectories lead to the idea that the atom has an electrically charged, relatively massive nucleus. On the diagram, draw such a trajectory for the alpha particle B. (1) (iii) Describe briefly how these trajectories lead to the idea of the nuclear model of the atom. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (4) 3. (a) Carbon-14 is a radioactive isotope with a half-life of 5500 years. It is produced in the atmosphere by neutron bombardment of nitrogen. The equation for this reaction is: 14 7 (i) N 01 n 146 C X. Explain what is meant by isotopes. ......................................................................................................................... ......................................................................................................................... (1) (ii) Identify the particle X. ......................................................................................................................... (1) (b) Each gram of a living tree contains approximately 4 1010 atoms of carbon-14. On the axes below, draw a graph to show the variation with time of the number of carbon-14 atoms in one gram of wood from a tree. Your graph should indicate the number of atoms for a period of 1.8 104 years after the tree has died. (Half-life of carbon-14 = 5500 years) /11 (3) 3 (c) The activity of a radioactive sample is proportional to the number of atoms in the sample. The activity per gram of carbon from a living tree is 9.6 disintegrations per minute. The activity per gram of carbon in burnt wood found at an ancient campsite is 1.9 disintegrations per minute. (i) Estimate the number of atoms of carbon-14 in the burnt wood. ......................................................................................................................... ......................................................................................................................... (1) (ii) From the graph you have drawn in (b), estimate the age of the burnt wood. ......................................................................................................................... (1) (d) In the decay of a Th-227 nucleus, a γ-ray photon is also emitted. Use the following data to deduce that the energy of the γ-ray photon is 0.667 MeV. mass of Th-227 nucleus = 227.0278 u mass of Ra-223 nucleus mass of helium nucleus = 223.0186 u = 4.0026 u energy of α-particle emitted =5.481 MeV unified atomic mass unit (u) = 931.5 MeV c–2 You may assume the Th-227 nucleus is stationary before decay and the Ra-223 nucleus has negligible kinetic energy. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) /5 4