DAR Request to Inject Cell Lines into Animals Form* *This form must
advertisement

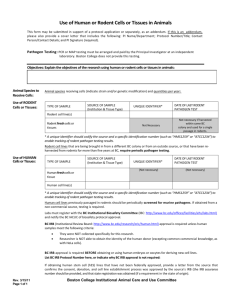
DAR Request to Inject Cell Lines into Animals Form* *This form must be approved by the GSU Attending Veterinarian prior to the use of said cell lines in animals. Please submit completed forms to the Associate to the Director) (mdavis1978@gsu.edu) Submission Date: _____________ PI Name Lab Contact PI E-mail Lab Contact E-mail PI Phone Number Lab Contact Phone Number Speedtype to be Charged (if testing is determined to be necessary) The injection of transplantable tumors, hybridomas, cultured cell lines, or other biological materials into rodents can pose a health risk to animals and personnel. These biological materials have been a source of mouse hepatitis virus, mousepox, and other significant disease agents at research facilities. Moreover, rodent pathogens can be carried and propagated by non-rodent (e.g. human) cell lines when these cell lines have been propagated in rodents or rodent biological materials. Consistent with GSU IACUC Policy, rodent cell lines (as well as non-rodent cell lines propagated in rodents or using rodent biological materials) must be evaluated for rodent pathogenic microorganisms by polymerase chain reaction (PCR) prior to their being injected into an animal. Approval for the use of such cell lines in animals housed at GSU will only be given after the Attending Veterinarian has assessed the test results to determine their adequacy. Some vendors of cell lines will already have screened the cells in which case additional testing may not be necessary. Should testing be necessary, the PCR assay requires 2 vials of each sample with a minimum of 1 x 107 cells/vial and a turnaround time of 7-10 days. Please fill out the table below for each cell line you plan to use in animals. The cost of this testing will be paid by the Principal Investigator. Cell Line Name Vendor Cell Line # (if such designation exists) Do you have record of this cell line having been previously tested for rodent pathogens? (Y or N) If Yes, please provide test results. Please note that individual cell lines must be approved for in vivo use by specific IACUC-approved protocols. _______________________________ _________________ University Veterinarian Signature Date