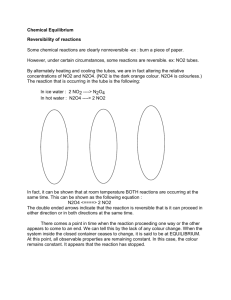
Chapter 15 Chemical Equilibruim
advertisement

Chapter 15 Chemical Equilibruim
Weak electrolytes don’t 100% dissociate in water
CH3COOH(aq) CH3COO- (aq) + H+ (aq)
The above reaction is in chemical equilibrium ( m); as we discussed in Chapter 4, the
double arrow indicates that the forward and reverse processes are occurring.
physical m;
H2O(l) H2O(g)
the above process represents the equilibrium that occurs at the boiling point of
water.
Chemical m
N2O4(g) 2NO2(g)
in terms of above reaction
N2O4(g) 2NO2(g)
forward
(rate)for = kfor * [N2O4]
backward
2 NO2(g) N2O4(g)
(rate)back = kback * [NO2]2 elementary reactions.
(rate)for = (rate)back
2
[N2 O 4 ] k for
= [NO 2 ]2 k back
[NO 2 ]2
=
[N2 O 4 ]
k for
k back
=
= K
where K = the reaction equilibrium constant
Alternatively look at the following data
H2 (g) + I2 (g) 2HI (g)
[ HI ] 2
define K
[ H2 ][ I2 ]
[H2]
[I2]
[HI]
[H2]eq
[I2]eq
[HI]eq
0.638
0.610
0.523
0.277
0.570
0.280
0.479
0.179
0
0
0
0
0.165
0.300
0.130
0.114
0.0978
0.0179
0.086
0.0165
0.945
0.540
0.786
0.326
Note: K = [HI]2/([I2] [H2]) is approximately constant
while [HI]/ ([I2][H2]) can vary by quite a bit
for any general reaction,
aA + bB
cC + dD
[C ]c [D]d
K
[ A]a [B] b
also known as the law of mass action.
{H2]eq
([I2][H2])eq
58.6
100.0
70.3
174
K
55.3
55.0
55.3
56.3
3
Examine the Magnitude of K
1.
K is very large >> 1
e.g. 203(g) 302(g)
K = [O2]3/[O3]2 = 2.54 * 1012
K is large, the reactants side is much less favoured, i.e., reaction mixture at
equilibrium contains primarily products.
2.
K << 1
e.g. Cl2(g) 2Cl(g)
K = [Cl]2/[Cl2] = 1.4 * 10-38
at m, there will be a much greater amount of reactant than product in the m
mixture.
3.
K~1
e.g.
CO(g) + H2O(g) CO2 (g) + H2(g)
[H2 ][CO2 ]
K
[CO][H2O]
= 5.10; T = 830C
the composition of the m mixture will be comparable as far as the concentration
of products and reactants is concerned.
Expressing K
Homgeneous systems
gas phase reaction
2 NO2(g) N2O4(g)
Kc = [NO2]2/N2O4]
Kc => moles/L
we could express the equilibrium constant in terms of the pressure of the
reactants and products
4
Kp = (PNO2 )2/ PN2O4
PNO2 ; PN2O4
are the partial pressures of NO2 and N2O4, respectively.
We find that Kc Kp in general, but they should be related somehow.
Relationship between - Kc and Kp
look at the following gas phase reaction
w X (g) y Z (g)
Kc = [Z]y/[X]w
but M = (moles of gas) / V
y
[Z ]
Kc =
[ X ]x
but M =
mol
V
nx
nz
Pz
Px
=
; [Z] =
=
v
RT
v
RT
[ n2 / V] y
Ideal gas eq.[ X ]
[Z] y
=
[X] w
[n x / V] w
subst into Kc exp ression
Pz y
(RT ) y
(Pz )y
Px w
( w-y )
=
w =
w * RT
(RT )
(Px )
but
(Pz )y
(Px )w
= K p K p * RT(w - y) = Kc
K p = Kc * RT
( y -w )
5
note y-w ng
Kp = Kc (RT)ng
R = 0.08206 Latm / (K mol) since most of our pressures will be in atmospheres.
6
Example
The reaction of N204(g) 2NO2(g) has a Kp of 4.3atm at 350 K. Calculate Kc at 350K
Kp = 4.3atm = Kc * (RT)ng ng = +1
4.3atm = Kc * 0.08206 L atm / (K mol) x 350 K
4.3
mol
0.08206 * 350
L
Kc = 0.150mol / L
Kc =
So far we have both with homogenous systems
CaCO3 (s) CaO (s) + CO2 (g)
Kc = [CO2(g)][CaO(s)] / [CaCO3(s)]
but CaO and CaCO3 are pure solids
we can treat their “concentration” as being constant
Kc [Ca CO3(s)] / [CaO (s)] = [CO2(g)] = Kc
the m process depends on the concentration of CO2(g) only!!!
Another example
TiCl4 (l) + O2(g) TiO2(g) + 2Cl2(g)
2
Kc =
[Cl2 (g) ] * [TiO2 (g)]
[TiCl 4 ( )][O2 (g)]
TiO2(s) and TiCl4 (l) are pure substances, their molar concentration is constant
7
2
]
[Cl
(g)
2
Kcl = Kc * [TiCl 4 ( )] / [TiO2 (s)] =
[O2 (g)]
Why do we say that their “molar concentration” (solids and liquids) is constant?
Mole / V density / molar mass
both are constants whether or
not we have 1,3,5, 1000g etc.
Dissociation of a weak acid (or base) in water.
NH 3 (aq) + H 2 O( ) NH+4 (aq) + OH - (aq)
+
Kc
[ NH4 (aq)][ OH - (aq)]
=
[ NH 3 (aq)][ H 2 O( )]
[H2O(l)] is essentially constant
+
[ NH 4 (aq)][ OH - (aq)]
K c * [ H 2 O( )] =
[ NH 3 (aq)]
K c K b the base disociation constant .
A similar constant can be defined for a weak acid, Ka acid dissociation
8
Example
The m constant for the following reaction,
CO2(g) + H2(g) CO(g) + H2O(g)
is equal to 0.11 at 700K. If we obtain the m concentrations we find that we have 0.20
mol of both CO(g) and H2O(g) and has 0.59 mol of CO2(g) (in a 1.00 L container),
calculate the concentration of H2(g).
K =
[CO][ H 2 ]
(0.20)(0.20)
=> 0.11 =
[ CO2 ][ H 2 ]
(0.59)[ H 2 ]
2
(0.20 )
=> [ H 2 (g)] =
(0.59(0.11)
0.62mol
Equilibria for Multiple reactions.
N2 (g) + O 2 (g) 2NO(g)
(K p )1 =
PNO 2
PN 2 PO 2
P
=
2
2NO(g) + O 2 (g) 2 NO2 (g)
overall
N2 (g) + 202 (g) 2 NO2 (g)
but we notice
(K p )2
NO2
PNO PO 2
(K p )overall =
P NO 2 2
PN 2 PO 2
9
PNO 2 2
PN 2 PO2
2
2
PNO 2
*
PN 2 PO 2 PNO 2 * PO 2
2
PNO
=
(K p ) ov erall = (K p )1 * (K p )2 => a general rule
if a reaction can be expressed as the sum of one or more reactions, then the overall
equilibrium constant is the product of the K1s of the individual reactions.
Look at the following reaction
N2O4 (g) 2NO2(g) (Kp)1 = (PNO2)2/ PN2O4
turn it around 2 NO2(g) N2O4(g)
(Kp)2 = PN2O4/(PNO2)2
note (Kp)2 = 1/(Kp)1
if we reverse a reaction, the m constant for the reversed reaction is the inverse
of the m constant in the originate direction
Multiplying a reaction
N2O4 (g) 2 NO2(g)
(Kc)1 = [NO2]2/ [N2O4]
multiply reaction by 3 => 3N2O4(g) 6NO2(g)
(Kc)2 = [NO2(g)]6/[N2O4(g)]3 = ([NO2(g)]2/[N2O4(g)])3
Relationship between Chemical m and chemical kinetics
We have already stated that within an equilibrium mixture, the microscopic recount, the
forward and reverse i.e., the forward and reverse reactions occur at equal rates (note
look at the “double arrow”)
Example
Look at the reaction of
2NOCl(g) 2NO(g) + Cl2(g)
10
Examine both the forward and reverse reactions.
2NOCl(g)
2NO(g) + Cl2(g)
Cl2 (g) + 2NO(g) 2NOCl(g)
(rate)for = kfor [NOCl]2
(rate)rev =krev [NO]2[Cl2]
at m (rate)for =(rate)rev
kfor [NOCl]2 = krev [NO]2[Cl2]
kfor / krev = [NO]2[Cl2]/[NOCl]2 Kc
VERY IMPORTANT
FOR ELEMENTARY REACTIONS if the value of the m constant is known as well
as a rate constant (where kfor or krev), the other rate constant may be easily found
e.g. for the above reaction, Kc = 1.7 * 10-4 mol/L
we are given kfor = 7.8 *10-2/ (Ms) calculate krev
Kc
= kfor / krev
krev = kfor /Kc
= 7.8*10-2 /(M s) / 1.7*10-4 mol/L
= 4 * 10-2/ (M s)
krev = 4.6 * 10+2/ (Ms)
What does the m constant tell us?
We can predict the direction of a chemical reaction
e.g. I2(g) + H2(g) 2 HI (g)
[I2]o = 0.277 ; [H2]o = 0.396; [HI]o = 1.27
define Qc the reaction quotient.
11
[ HI ] o2
Qc
[ H2 ]o [ I2 ]o
[1.27 ] 2
Qc
[ 0 .396 ][ 0 .277 ]
Qc = 14.7 (note Kc = 55)
Note a)
Qc < Kc => the ratio of the initial products is too small reactants must be
converted to products
b) [HI]o = 1.98; [I2]o = 0.026; [H2]o = 0.117
[1.98 ] 2
Qc
[ 0 .117 ][ 0 .0 .026 ]
Qc > Kc some products will have to be converted to reactants as the ratio of
products to reactants is too small
c) Qc = Kc the system is at m
e.g. N2(g) + 3H2 (g) 2NH3(g)
Kc =
[ NH 3 ]
2
[ N 2 ][ H 2 ]
3
= 0.65 [ N 2 ] = 0.0627 [ NH 3 ] = 1.27 * 10 -5
[H2] = 0.00877
2
2
[ NH 3 ] 0
(1.27 * 10 -5 )
-3
Qc =
3 =
3 = 3.81 * 10
[ N 2 ] 0 [H ] 0
0.0627)(0.00877 )
note Qc < K c
Handling m calculations
lets look at the following example
12
Reaction 2NO2(g) N2O4(g) K= 216
[NO2] = 0.267 mol/L
Equilibrium Data Table
K expression Kc = [N2O4]/[NO2]2
Start
Change
m
[NO2]
0.267
-2x
0.267-2x
[N2O4]
0
+X
X
K = [N2O4]/[NO2]2 = [X]/[0.267-2x]2
216 * (0.267-2x)2 = x; 216 * (0.267-2x) (0.267-2x) = x
216 * (0.0713 -1.058 x + 4x2) = X
15.4 - 231 X + 864 x2 = x
15.4 - 232 X + 864x2 = 0
This is a quadratic equation; this can be solved using the quadratic formula.
- b+ _ b2 - 4ac
x =
2ac
For this particular equation
a = 864; b = 232;c = 15.4
9
From the quadratic equation, we will obtain two roots.
Root #1: x (1) = 0.146; Root #2: x (2) = 0.122
Whenever we use the quadratic equation in a chemical equilibrium problem, we will find
that one and only one root is a valid solution to the equilibrium problem (i.e., the other
root gives us a solution that is physically meaningless).
Which root would we choose in this case?
13
[NO2]eq = 0.267 - 2x
If we use the value of Root #1
[NO2] at m = 0.267 - 2 (0.146) = -0.025 M < 0.
This is an impossible situation.
Therefore, we find that Root #1 is not acceptable ([NO2] = -0.025??)
Root #2 x(2) = 0.122 is the answer that makes physical sense.
check [NO2] = 0.023 = [0.267 - 2*(0.122)]
[N2O4] = 0.122.
When we substitute the equilibrium concentrations into the K expression, we
obtain the following.
K = [0.122]/[0.023]2 = 218 (very close)
14
Another example
N2(g) + 3H2(g) 2NH3(g) Kp = 4.31 * 10-4; T = 473K
PN2 (g) = 1.214 atm
PH2 (g) = 0.526 atm
Equilibrium Data Table
K expression
PN2/atm
1.214
-x
1.214 - x
Start
Change
m
K
K
2
PNH
3
PN2 PH32
2
PNH
3
PN2 PH32
PH2/atm
0.526
-3x
0.526 - 3x
PNH3/atm
0
+2x
2x
2x 2
4
3 4.1x10
=
1214
.
x 0.526 3 x
Note that this is a very complicated expression to solve. But we can simplify matters
considerably!!
note value of Kp <<<1 which indicates that the reaction mixtures is composed primarily of
reactants at equilibrium. This indicates that x is very small. Therefore, we can assume
the following.
assume (1.214-x) 1.214
NOTE: IN PRACTICE, IF THE VALUE OF THE EQUILIBRIUM CONSTANT
FOR THE REACTION IS, K < 0.0010 , AN ASSUMPTION MAY BE
POSSIBLE.
We would also assume 0.526-3x 0.521
K
2
PNH
3
PN2 PH32
(2x )2
=
1214
0.526 3
.
0.7615 * 10-4 = (2x)2 take the square root of both sides.
15
2x = 0.873 * 10-2
or x = 4.36 * 10-3 atm
Check our assumptions
1.214 - 4.36 x 10-3 = 1.210
0.526 - 3 (4.36 x 10-3) = 0.513
this is a good approximation IF AND ONLY IF
nX/ Initial pressure < 5%
Check
4.36 x 10-3 / 1.214 * 100% = 0.359 %
3(4.36 x 10-3) / 0.526 * 100%
= 0.0131/0.526 *100% = 2.51%
Note: Both assumptions are valid; therefore, x = 0.00436 atm is a reasonable
approximation to the amount of N2 (g) that reactas to form NH3 (g) at
equilibrium.
Factors that Affect m
m is a delicate balance. Note that we can change conditions such as [reactants].
reactants (or products), temp., pressure (external) and volume; how do these affect m.
Le Chatelier’s Principle
If a system in chemical m is subjected to an external stress, the system adjusts itself in
such a way that the stress is relieved
Concentration Changes
e.g. FeSCN2+ (aq) Fe3+(aq) + SCN- (aq)
red
yellow colourless
add Fe(NO3) (aq) to the equilibrium system, this will place a stress on the RHS of the
reaction, and the equilibrium system will be pushed to the reactants side (i.e., there will
be an increase in the red colour due to the increase in the concentration of the
FeSCN2+ complex.
16
add SCN- (aq) to the system as NaSCN (aq), we also see that the m shifts to left
since the “stress” is on the right side of the reaction (similar to the Fe3+ (aq) addition
above).
Add a species that reacts strongly with Fe3+ (aq).
(e.g. Na2C2O4(aq)).
Na2C2O4 (aq)
furnishes
C2O42- (aq) ions which react strongly with the Fe3+ ion.
3+
Hence, this in effect removes Fe from the equilibrium system. A stress is placed on the
left hand side of the reaction, and more FeSCN2+ must dissociate to produce Fe3+ (the
red colour fades).
Change in Pressure or Volume
2NO2 N2O4
Kc = 216 = [N2O4]/[NO2]2
Increase external pressure => this favours the combination of 2NO2 to N2O4 since
P(RHS) < P(LHS) for equal numbers of moles of gas; the reaction will go in the direction
of the fewest number of moles of gas.
The effect of the stress on the equilibrium is dependent on the value of ng = n (gaseous
products) - n(gaseous reactants).
P
ng
+
+
-
Direction of Shift
to the left
to the right
to the right
to the left
What if (n (products) - n(reactants)) = 0?
THERE IS NO EFFECT ON THE EQUILIBRIUM POSITION OF THE REACTION!!
The same effects are observed for decreasing volumes of the container, since P 1/V
decrease V and we increase P.
What if we decrease the pressure for the above reaction? The reaction is now going to
go in the direction of increasing number of moles of gas (the same effect is observed for
an increase in volume since V means P)
17
Can we change the pressure without changing the volume? If we add an inert gas (a
gas that does not participate in the equilibrium reaction). The noble gases are good
candidates (He, Ne, Ar etc.).
e.g. 2NO2 N2O4
P(RHS) < P(LHS) (V constant => n(RHS) <n(LHS)).
If we add He to the system, we increase the total pressure, PT, but the partial
pressures of the equilibrium gases PN204 and PNO2 don’t change.
THERE IS NO EFFECT ON THE m POSITION ALTHOUGH WE WOULD
THINK THERE WOULD BE ONE.
Temperature Changes
2NO2(g) N2O4(g)
rxnH = -58.0 KJ
the simplest way to attack this is to rewrite the reaction as follows
2NO2(g) N2O4(g) + heat (i.e., heat is evolved)
when we increase T, we increase the “quantity of heat” (at const. V of
course).
the reaction is pushed to the left according to LeChatelier’s principle.
What about the following?
N2F4(g) 2NF2(g) rxnH = 38.5kJ
rxnH > 0 means heat is absorbed
rewrite with heat on LHS of the reaction.
heat + N2F4(g) 2NF2(g)
If we heat the reaction, the stress is placed on the LHS and according to the LeChatelier
principle, the reaction m position would go from left to right (i.e. formation of NF2 is
favoured).
18
Catalysts => CATALYSTS SPEED UP RATES OF CHEMICAL REACTIONS!
They have no effect on the position of an m. Why?
C5H10 (g) + H2 (g) C5H12(g)
In the hydrogenation reaction, the catalyst (Pt) speeds up the rates of both the forward
and reverse reactions equally. The m concentrations are achieved faster, but they are
still the concentrations predicted by the m constant for the reaction!