Supplementary Information (doc 53K)
advertisement

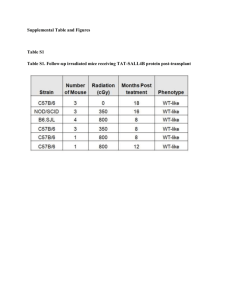
Suzuki et al. 1 Supplemental Information Generation of Engraftable Hematopoietic Stem Cells from Induced Pluripotent Stem Cells by way of Teratoma Formation Nao Suzuki1, 3*, Satoshi Yamazaki1, 2*, Tomoyuki Yamaguchi1, 2, Motohito Okabe1, Hideki Masaki2, Satoshi Takaki3, Makoto Otsu1, and Hiromitsu Nakauchi1, 2 * These authors contributed equally to this work. 1 Division of Stem Cell Therapy, Center for Stem Cell Biology and Regenerative Medicine, Institute of Medical Science, University of Tokyo, Tokyo 108-8639, Japan 2 Japan Science Technology Agency, Exploratory Research for Advanced Technology (ERATO) Nakauchi Stem Cell and Organ Regeneration Project, Tokyo 108-8639, Japan 3 Department of Immune Regulation Research Institute, National Center for Global Health and Medicine, Chiba 272-8516, Japan Contains 5 Supplemental Figures and figure legends and one Supplemental Table. 1 Suzuki et al. 2 Legends for Supplemental Figures Figure S1. Engraftable HSCs were induced from LG-iPSCs through teratoma formation (a) Phase change of LG-iPSC-derived GFP+ CD45+ cells in PB of teratoma-bearing mice through administration of cytokines and OP9 cells. (b) Analysis of BM cells of teratoma-bearing mice through administration of cytokines and OP9 cells 12 weeks post iPSC injection. (c, d) BM transplantation assay. BM cells of teratoma-bearing mice were transplanted into irradiated mice. (c) The chimerism of LG-iPSC-derived GFP+ hematopoietic cells/CD45+ cells in spleen and bone marrow of recipient mice (n=4) 12 weeks post primary transplantation. (d) The percentage of GFP+ cells/HPCs (Lin-) or HSCs (CD34-KSL) in BM of recipient mice (n=4) 12 weeks post primary transplantation. Error bar represent SD. Fugure S2. Engraftable HSCs were induced from G-iPSCs through teratoma formation (a) Phase change of G-iPSC-derived GFP+ CD45+ cells in PB of teratoma-bearing mice through administration of cytokines and OP9 cells. (b) Analysis of BM cells of teratoma-bearing mice through administration of cytokines and OP9 cells 12 weeks post iPSC injection. (c) The chimerism of G-iPSC-derived GFP+ hematopoietic cells/CD45+ cells in spleen and BM of recipient mice (n=6) 12 weeks post primary transplantation. (d) The percentage of GFP+ cells/HPCs (Lin-) or HSCs (KSL; 2 Suzuki et al. 3 short-term, CD34-KSL; long-term) in spleen and BM of recipient mice (n=6) at 12 weeks post primary transplantation. Error bar represent SD. (e) Blood count of recipient mice at 12 months after first transplantation and age matched wild type B6 mice (n=4). WBC, white blood count; RBC, red blood count; PLT, platelet; red lines represent the minimum value; WT, Wild type mice; RC, recipient mice. Figure S3. Establishment of gene-corrected mγc-iPSCs from X-SCID mice Expression of GFP and murine gamma chain (γc) in mγc-iPSCs. B6 iPSCs were used as a negative control. Figure S4. Induction of engraftable HSCs from hiPSCs through teratoma formation (a) Numbers of mice in which hiPSC-derived cells were detected in teratoma-bearing mice or recipient mice transplanted with hiPSC-derived HSCs. (b-d) Immunostaining of human β-globin in hiPSC-derived glycophorinA+ erythrocytes isolated from PB of recipient mice (b) and human erythrocytes in PB (c), and mouse erythrocytes in PB (d). (e-g) Wright-Giemsa stains of cytospin preparations of hiPSC-derived glycophorinA+ erythrocytes isolated from PB of recipient mice (e) and human erythrocytes in PB (f), and mouse erythrocytes in PB (g). Scale bars, 10μm. Figure S5. iPSC-derived hematopoietic cells including HSCs and HSC niche components were 3 Suzuki et al. 4 coexist in teratomas (a-c) Immunostaining of CD45, c-Kit and HSC niche-cell markers GFAP(a), osteocalcin (b), and VE-cadherin (c) in G-iPSC-derived teratomas. Scale bar, 75 μm. (d, e) Immunostaining of hCD45, hCD34, and HSC niche-like cells in hiPSC-derived teratoma sections. HSC niche-like cell markers were osteocalcin (a), VE-cadherin (b). Scale bar, 30μm. Arrowheads indicate CD45+ CD34+ cells nearby HSC niche-like cells. Table S1. Chimerism of iPS-derived hematopoietic cells in recipient mice 12 weeks after bone marrow transplantation T cell chimerism is the percentage of GFP+ CD3+/CD45+ cells, B cell chimerim is the percentage of GFP+ B220+/CD45+ cells, and myeloid chimerism is the percentage of GFP+ Gr-1+ Mac-1+ /CD45+ cells. (Mean ± s.e.m.; LG-iPSCs group, n=4; G-iPSCs group, n=6.) iPSCs Peripheral blood Spleen Bone marrow T cell B cell Myeloid T cell B cell Myeloid T cell B cell Myeloid LG-iPSCs 20.9 ± 5.1 25.7 ± 5.7 26.5 ± 8.2 15.0 ± 0.1 24.9 ± 0.9 21.8 ± 0.5 1.3 ± 0.1 6.0 ± 0.4 48.0 ± 1.6 G-iPSCs 0.3 ± 0.2 2.4 ± 1.6 2.5 ± 1.1 0.3 ± 0.1 1.8 ± 0.1 0.3 ± 0.1 0.2 ± 0.1 0.7 ± 0.1 2.4 ± 1.2 4




![Historical_politcal_background_(intro)[1]](http://s2.studylib.net/store/data/005222460_1-479b8dcb7799e13bea2e28f4fa4bf82a-300x300.png)